Enzyme Inhibition: Mechanisms and Scope
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allosteric Regulation in Drug Design
Mini Review Curr Trends Biomedical Eng & Biosci Volume 4 Issue 1 - May 2017 Copyright © All rights are reserved by Ashfaq Ur Rehman DOI: 10.19080/CTBEB.2017.04.5555630 Allosteric regulation in drug design Ashfaq Ur Rehman1,2*, Shah Saud3, Nasir Ahmad4, Abdul Wadood2 and R Hamid5 1State Key Laboratory of Microbial Metabolism, Department of Bioinformatics and Biostatistics, China 2Department of Biochemistry, Abdul Wali Khan University Mardan, Pakistan 3Laboratory of Analytical Biochemistry and Bio separation, Shanghai Jiao Tong University, China 4Department of Chemistry, Islama College University Peshawar, Pakistan 5Department of Bioinformatics, Muhammad Ali Jinnah University Islamabad, Pakistan Submission: May 02, 2017; Published: May 23, 2017 *Corresponding author: Ashfaq Ur Rehman, State Key Laboratory of Microbial Metabolism, Department of Bioinformatics and Biostatistics, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China, Tel: ; Fax: 86-21-34204348; Email: Abstract mechanism, which are initiated through attachment of ligand or inhibitors with the protein or enzymes other than active (orthosteric) sites. ThisProtein mini review and enzymes involved play mechanism, significant types roles and in importancebiological processes of allosteric of all regulations living organisms; in drug theirdesign functions process. are regulated through allosteric Keywords: Allosteric, Activator: Drug design Introduction and ultimately cause disease. While various biological processes expressed the control at different points in life time of protein function is pivotal. As all the cell processes are under carful For the survival of all organisms the significance of protein included regulation of gene expression, translation into protein control and if not properly controls this leads to the abnormality through control of activity and at last degradation of protein [1]. -

Tyrosine Kinase – Role and Significance in Cancer
Int. J. Med. Sci. 2004 1(2): 101-115 101 International Journal of Medical Sciences ISSN 1449-1907 www.medsci.org 2004 1(2):101-115 ©2004 Ivyspring International Publisher. All rights reserved Review Tyrosine kinase – Role and significance in Cancer Received: 2004.3.30 Accepted: 2004.5.15 Manash K. Paul and Anup K. Mukhopadhyay Published:2004.6.01 Department of Biotechnology, National Institute of Pharmaceutical Education and Research, Sector-67, S.A.S Nagar, Mohali, Punjab, India-160062 Abstract Tyrosine kinases are important mediators of the signaling cascade, determining key roles in diverse biological processes like growth, differentiation, metabolism and apoptosis in response to external and internal stimuli. Recent advances have implicated the role of tyrosine kinases in the pathophysiology of cancer. Though their activity is tightly regulated in normal cells, they may acquire transforming functions due to mutation(s), overexpression and autocrine paracrine stimulation, leading to malignancy. Constitutive oncogenic activation in cancer cells can be blocked by selective tyrosine kinase inhibitors and thus considered as a promising approach for innovative genome based therapeutics. The modes of oncogenic activation and the different approaches for tyrosine kinase inhibition, like small molecule inhibitors, monoclonal antibodies, heat shock proteins, immunoconjugates, antisense and peptide drugs are reviewed in light of the important molecules. As angiogenesis is a major event in cancer growth and proliferation, tyrosine kinase inhibitors as a target for anti-angiogenesis can be aptly applied as a new mode of cancer therapy. The review concludes with a discussion on the application of modern techniques and knowledge of the kinome as means to gear up the tyrosine kinase drug discovery process. -

DNA Breakpoint Assay Reveals a Majority of Gross Duplications Occur in Tandem Reducing VUS Classifications in Breast Cancer Predisposition Genes
© American College of Medical Genetics and Genomics ARTICLE Corrected: Correction DNA breakpoint assay reveals a majority of gross duplications occur in tandem reducing VUS classifications in breast cancer predisposition genes Marcy E. Richardson, PhD1, Hansook Chong, PhD1, Wenbo Mu, MS1, Blair R. Conner, MS1, Vickie Hsuan, MS1, Sara Willett, MS1, Stephanie Lam, MS1, Pei Tsai, CGMBS, MB (ASCP)1, Tina Pesaran, MS, CGC1, Adam C. Chamberlin, PhD1, Min-Sun Park, PhD1, Phillip Gray, PhD1, Rachid Karam, MD, PhD1 and Aaron Elliott, PhD1 Purpose: Gross duplications are ambiguous in terms of clinical cohort, while the remainder have unknown tandem status. Among interpretation due to the limitations of the detection methods that the tandem gross duplications that were eligible for reclassification, cannot infer their context, namely, whether they occur in tandem or 95% of them were upgraded to pathogenic. are duplicated and inserted elsewhere in the genome. We Conclusion: DBA is a novel, high-throughput, NGS-based method investigated the proportion of gross duplications occurring in that informs the tandem status, and thereby the classification of, tandem in breast cancer predisposition genes with the intent of gross duplications. This method revealed that most gross duplica- informing their classifications. tions in the investigated genes occurred in tandem and resulted in a Methods: The DNA breakpoint assay (DBA) is a custom, paired- pathogenic classification, which helps to secure the necessary end, next-generation sequencing (NGS) method designed to treatment options for their carriers. capture and detect deep-intronic DNA breakpoints in gross duplications in BRCA1, BRCA2, ATM, CDH1, PALB2, and CHEK2. Genetics in Medicine (2019) 21:683–693; https://doi.org/10.1038/s41436- Results: DBA allowed us to ascertain breakpoints for 44 unique 018-0092-7 gross duplications from 147 probands. -

Arginine Kinase: a Crystallographic Investigation of Essential Substrate Structure Shawn A
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2006 Arginine Kinase: A Crystallographic Investigation of Essential Substrate Structure Shawn A. (Shawn Adam) Clark Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES ARGININE KINASE; A CRYSTALLOGRAPHIC INVESTIGATION OF ESSENTIAL SUBSTRATE STRUCTURE BY SHAWN A. CLARK A Dissertation submitted to the Department of Chemistry and Biochemistry In partial fulfillment of the requirements for the Degree of Doctor of Philosophy Degree Awarded: Fall Semester, 2006 The members of committee approve the dissertation of Shawn A. Clark defended on 11/2/2006 __________________ Michael Chapman Professor Co-Directing Dissertation __________________ Tim Logan Professor Co-Directing Dissertation __________________ Ross Ellington Outside Committee Member __________________ Al Stiegman Committee Member _________________ Tim Cross Committee Member Approved: ________________________________________ Naresh Dalal, Department Chair, Department of Chemistry & Biochemistry The Office of Graduate Studies has verified and approved the above named committee members. ii ACKNOWLEDGEMENTS The voyage through academic maturity is full of numerous challenges that present themselves at many levels: physically, mentally, and emotionally. These hurdles can only be overcome with dedication, guidance, patience and above all support. It is for these reasons that I would like to express my sincerest and deepest admiration and love for my family; my wife Bobbi, and my two children Allexis and Brytney. Their encouragement, sacrifice, patience, loving support, and inquisitive nature have been the pillar of my strength, the beacon of my determination, and the spark of my intuition. -

The Self-Inhibitory Nature of Metabolic Networks and Its Alleviation Through Compartmentalization
ARTICLE Received 30 Oct 2016 | Accepted 23 May 2017 | Published 10 Jul 2017 DOI: 10.1038/ncomms16018 OPEN The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization Mohammad Tauqeer Alam1,2, Viridiana Olin-Sandoval1,3, Anna Stincone1,w, Markus A. Keller1,4, Aleksej Zelezniak1,5,6, Ben F. Luisi1 & Markus Ralser1,5 Metabolites can inhibit the enzymes that generate them. To explore the general nature of metabolic self-inhibition, we surveyed enzymological data accrued from a century of experimentation and generated a genome-scale enzyme-inhibition network. Enzyme inhibition is often driven by essential metabolites, affects the majority of biochemical processes, and is executed by a structured network whose topological organization is reflecting chemical similarities that exist between metabolites. Most inhibitory interactions are competitive, emerge in the close neighbourhood of the inhibited enzymes, and result from structural similarities between substrate and inhibitors. Structural constraints also explain one-third of allosteric inhibitors, a finding rationalized by crystallographic analysis of allosterically inhibited L-lactate dehydrogenase. Our findings suggest that the primary cause of metabolic enzyme inhibition is not the evolution of regulatory metabolite–enzyme interactions, but a finite structural diversity prevalent within the metabolome. In eukaryotes, compartmentalization minimizes inevitable enzyme inhibition and alleviates constraints that self-inhibition places on metabolism. 1 Department of Biochemistry and Cambridge Systems Biology Centre, University of Cambridge, 80 Tennis Court Road, Cambridge CB2 1GA, UK. 2 Division of Biomedical Sciences, Warwick Medical School, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK. 3 Department of Food Science and Technology, Instituto Nacional de Ciencias Me´dicas y Nutricio´n Salvador Zubira´n, Vasco de Quiroga 15, Tlalpan, 14080 Mexico City, Mexico. -

Plant Protease Inhibitors: a Defense Strategy in Plants
Biotechnology and Molecular Biology Review Vol. 2 (3), pp. 068-085, August 2007 Available online at http://www.academicjournals.org/BMBR ISSN 1538-2273 © 2007 Academic Journals Standard Review Plant protease inhibitors: a defense strategy in plants Huma Habib and Khalid Majid Fazili* Department of Biotechnology, The University of Kashmir, P/O Naseembagh, Hazratbal, Srinagar -190006, Jammu and Kashmir, India. Accepted 7 July, 2007 Proteases, though essentially indispensable to the maintenance and survival of their host organisms, can be potentially damaging when overexpressed or present in higher concentrations, and their activities need to be correctly regulated. An important means of regulation involves modulation of their activities through interaction with substances, mostly proteins, called protease inhibitors. Some insects and many of the phytopathogenic microorganisms secrete extracellular enzymes and, in particular, enzymes causing proteolytic digestion of proteins, which play important roles in pathogenesis. Plants, however, have also developed mechanisms to fight these pathogenic organisms. One important line of defense that plants have to fight these pathogens is through various inhibitors that act against these proteolytic enzymes. These inhibitors are thus active in endogenous as well as exogenous defense systems. Protease inhibitors active against different mechanistic classes of proteases have been classified into different families on the basis of significant sequence similarities and structural relationships. Specific protease inhibitors are currently being overexpressed in certain transgenic plants to protect them against invaders. The current knowledge about plant protease inhibitors, their structure and their role in plant defense is briefly reviewed. Key words: Proteases, enzymes, protease inhibitors, serpins, cystatins, pathogens, defense. Table of content 1. -

Effect of Statins and ACE Inhibitors Alone and in Combination on Clinical Outcome in Patients with Coronary Heart Disease
Journal of Human Hypertension (2004) 18, 781–788 & 2004 Nature Publishing Group All rights reserved 0950-9240/04 $30.00 www.nature.com/jhh ORIGINAL ARTICLE Effect of statins and ACE inhibitors alone and in combination on clinical outcome in patients with coronary heart disease VG Athyros1, DP Mikhailidis3, AA Papageorgiou2, VI Bouloukos1, AN Pehlivanidis1, AN Symeonidis4 and M Elisaf5, for the GREACE Study Collaborative Group 1Atherosclerosis Unit, Aristotelian University, Hippocration Hospital, Thessaloniki, Greece; 2Department of Clinical Biochemistry, Royal Free Hospital, Royal Free and University College Medical School, Pond Street, London, UK; 32nd Propedeutic Department of Internal Medicine, Aristotelian University, Hippocration Hospital, Thessaloniki, Greece; 4Greek Society of General Practitioners, Thessaloniki, Greece; 5Department of Internal Medicine, Medical School, University of Ioannina, Greece We assessed the ‘synergy’ of statins and angiotensin- point in group A was 31%, (95% CI À48 to À6%, P ¼ 0.01) converting enzyme inhibitors (ACEI) in reducing vascu- in comparison to group B, 59% (95% CI À72 to À48%, lar events in patients with coronary heart disease (CHD). Po0.0001) to group C and 63% (95% CI À74 to À51%, The GREek Atorvastatin and CHD Evaluation (GREACE) Po0.0001) to group D. There was no significant Study, suggested that aggressive reduction of low difference in RRR between groups C and D (9%, CI density lipoprotein cholesterol to 2.59 mmol/l À27–10%, P ¼ 0.1). Other factors (eg the blood pressure) (o100 mg/dl) significantly reduces morbidity and mor- that can influence clinical outcome did not differ tality in CHD patients, in comparison to undertreated significantly between the four treatment groups. -

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -

Humanization and Directed Evolution of the Selenium-Containing Scfv
RSC Advances View Article Online PAPER View Journal | View Issue Humanization and directed evolution of the selenium-containing scFv phage abzyme Cite this: RSC Adv.,2018,8,17218 Yan Xu,a Pengju Li,a Jiaojiao Nie,a Qi Zhao, b Shanshan Guan,a Ziyu Kuai,a Yongbo Qiao,a Xiaoyu Jiang,a Ying Li,a Wei Li,a Yuhua Shi,a Wei Kongac and Yaming Shan *ac According to the binding site structure and the catalytic mechanism of the native glutathione peroxidase (GPX), three glutathione derivatives, GSH-S-DNP butyl ester (hapten Be), GSH-S-DNP hexyl ester (hapten He) and GSH-S-DNP hexamethylene ester (hapten Hme) were synthesized. By a four-round panning with a human synthetic scFv phage library against three haptens, the enrichment of the scFv phage particles with specific binding activity could be determined. Three phage particles were selected binding to each glutathione derivative, respectively. After a two-step chemical mutation to convert the serine residues of the scFv phage particles into selenocysteine residues, GPX activity could be observed and determined upto 3000 U mmolÀ1 in the selenium-containing scFv phage abzyme which was isolated by Creative Commons Attribution 3.0 Unported Licence. affinity capture against the hapten Be. Also the scFv phage abzymes elicited by different antigens displayed different catalytic activities. After a directed evolution by DNA shuffling to improve the affinity Received 31st March 2018 to the hapten Be, a secondary library with GPX activity was created in which the catalytic activity of the Accepted 3rd May 2018 selenium-containing scFv phage abzyme could be increased 17%. -
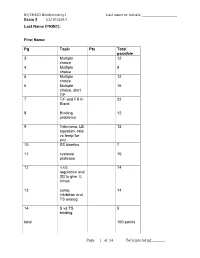
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

(12) United States Patent (10) Patent No.: US 6,387,890 B1 Christianson Et Al
USOO638789OB1 (12) United States Patent (10) Patent No.: US 6,387,890 B1 Christianson et al. (45) Date of Patent: May 14, 2002 (54) COMPOSITIONS AND METHODS FOR CA:87:6321 abs of Diss Abstr Int B 37(8) pp. 3927-8 by INHIBITING ARGINASE ACTIVITY Hartz. Albina et al., 1995, J. Immunol. 155:4391-4396. (75) Inventors: David Christianson, Media, PA (US); Bacon et al., 1988, J. Mol. Graph. 6:219–200. Ricky Baggio, Waltham; Daniel Baker et al., 1980, Fed. Proc., Fed. Am. Soc. Exp. Biol. Elbaum, Newton, both of MA (US) 39:1686. Baker et al., 1983, Biochemistry 22:2098-2103. (73) Assignee: Trustees of the University of Bergeron et al., 1987, J. Org. Chem. 52:1700–1703. Pennsylvania, Philadelphia, PA (US) Biancani et al., 1985, Gastroenterology 89:867-874. - 0 Boden, 1975, Synthesis 784. (*) Notice: Subject to any disclaimer, the term of this Boucher et al., 1994, Biochem. Biophys. Res. Commun. patent is extended or adjusted under 35 2O3:1614-1621. U.S.C. 154(b) by 0 days. Bringer et al., 1987, Science 235:458–460. Bringer et al., 1998, Acta Crystallogr. D54:905-921. (21) Appl. No.: 09/545,737 Campos et al., 1995, J. Biol. Chem. 270:1721–1728. Cavaili et al., 1994, Biochemistry 33:10652–10657. (22) Filed: Apr. 10, 2000 Chakder and Rattan, 1997, J. Pharmacol. Exp. Ther. 282:378-384. Related U.S. Application Data Chakder and Rattan, 1993, Am. J. Physiol. Gastrointest. (63) Continuation-in-part of application No. PCT/US98/21430, Liver Physiol. 264:G7-G12. filed on Oct.