Enzymes: the Biological Accelerators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -

In Vivo Kinetics of Thymidylate Synthetase Inhibition in 5-Fluorouracil- Sensitive and -Resistant Murine Colon Adenocarcinomas1
[CANCER RESEARCH 42, 450-456, February 1982) 0008-5472/82/0042-OOOOS02.00 In Vivo Kinetics of Thymidylate Synthetase Inhibition in 5-Fluorouracil- sensitive and -resistant Murine Colon Adenocarcinomas1 C. Paul Spears, Antranik H. Shahinian, Richard G. Moran, Charles Heidelberger,2 and Thomas H. Corbett Cancer Research Laboratories, University of Southern California Comprehensive Cancer Center, Los Angeles, California 90033 1C. P. S., A. H. S., C. H.]; Childrens Hospital of Los Angeles, Los Angeles, California 9002 7 [R. G. M.I and Southern Research Institute, Birmingham, Alabama 35255 [T. H. C.] ABSTRACT is inactivated rapidly in the presence of the 5-FUra metabolite, FdUMP, and CH2FH4, by the formation of an enzyme:FdUMP: The predictive utility of several biochemical parameters of 5- CH2FH4 covalently bonded ternary complex (10, 25, 43), from fluorouracil (5-FUra) action was evaluated in four murine co- which native TS is slowly released (11, 29, 48). Ionic adenocarcinomas: 5-FUra-sensitive Tumor 38 and 5- Evidence that TS inactivation is the critical therapeutic effect FUra-resistant Tumors 07/A, 51, and 06/A. Thymidylate syn- of 5-FUra has been largely indirect, because of difficulties in thetase (TS) was determined by a tritiated 5-fluoro-2'-deoxyu- the assay of low, growth-limiting levels of TS. Recent studies ridylate (FdUMP)-binding assay. Bolus 5-FUra (80 mg/kg, i.p.) using a sensitive tritium release TS assay (42), however, have administration caused in all tumors a rapid decrease in free TS indicated correlations between 5-FUra cytotoxicity and de levels. Only Tumor 38, however, showed inhibition of TS to creases in tumor TS activity (3, 12). -
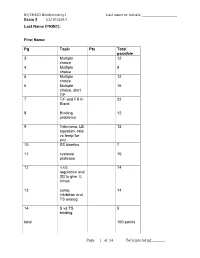
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

Optimal Scheduling of Methotrexate and 5-Fluorouracil in Human Breast Cancer1
[CANCER RESEARCH 42, 2081-2086, May 1982] 0008-5472/82/0042-0000$02.00 Optimal Scheduling of Methotrexate and 5-Fluorouracil in Human Breast Cancer1 Chris Benz, Tina Tillis, Ellen Tattelman, and Ed Cadman2 Departments of Medicine and Pharmacology, Yale School of Medicine, New Haven, Connecticut 06510 ABSTRACT tually die of disseminated disease (11, 25). The use of combi nation chemotherapy in disseminated breast cancer has im We have shown previously that methotrexate pretreatment proved objective response rates over single-agent therapy by of murine leukemia and human colon carcinoma cell cultures about 40%, yet there has been no improvement in overall results in augmented intracellular accumulation of 5-fluorour- survival and no clear superiority in palliative benefit over the acil metabolites. Both of these drugs are commonly used for use of sequential single-agent therapy (9, 18). These discour the treatment of women with breast cancer; thus, sequencing aging facts reflect the brevity of response durations following of methotrexate before 5-fluorouracil was evaluated in vitro systemic chemotherapy, 7 to 11 months, and low complete using a human mammary carcinoma cell line, 47-DN. Intracel response rates of about 15% (7). Perhaps more knowledgeable lular 5-fluorouracil accumulation was maximally increased 4- scheduling of multiple drugs given in combination will improve fold in cultures pretreated with 10 /ÕMmethotrexate for 24 hr. our therapeutic impact on breast cancer; basic laboratory This enhancement of 5-fluorouracil metabolism was associated studies may be able to provide the necessary rationale for with increased intracellular levels of 5-phosphoribosyl 1-pyro- devising such synergistic combinations. -

Study Protocol
Full title: A Sequential Phase I study of MEK1/2 inhibitors PD-0325901 or Binimetinib combined with cMET inhibitor PF-02341066 in Patients with RAS Mutant and RAS Wild Type (with aberrant c-MET) Colorectal Cancer Short title: MErCuRIC1: MEK and MET Inhibition in Colorectal Cancer Protocol Version & date: Version 9.0; 29Oct2018 Sponsor Protocol Number: OCTO_049 Ethics Number: 14/SC/1010 EudraCT Number: 2014-000463-40 Funding Reference Number 602901-2 Sponsored by the University of Oxford Funder: European Commission’s Seventh Framework Program (FP7) Confidentiality Statement This document contains confidential information that must not be disclosed to anyone other than the Sponsor, the Trials Office, the Investigator Team, host NHS Trust(s), regulatory authorities, and members of the Research Ethics Committee. MErCuRIC1_Protocol_V9.0_29Oct2018 Protocol Template V3.0_18Feb2013 Page 1 of 121 MErCuRIC1 Confidential GENERAL CONTACT INFORMATION Trial Office (OCTO) MErCuRIC Trial Office Oncology Clinical Trials Office (OCTO) Department of Oncology, The University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford Tel: +44 (0)1865 227194 Email: [email protected] Website: http://www.oncology.ox.ac.uk/research/oncology- clinical-trials-office-octo Chief Investigator Professor Mark Middleton Department of Oncology, University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford OX3 7LE Tel: +44 (0)1865 235 315 Email : [email protected] Investigator Professor Richard Wilson and -

Effects of Synergistic Inhibition on Α-Glucosidase by Phytoalexins In
biomolecules Article Effects of Synergistic Inhibition on α-glucosidase by Phytoalexins in Soybeans Hyeong-U Son 1 , Eun-Kyeong Yoon 1, Chi-Yeol Yoo 1, Chul-Hong Park 2, Myung-Ae Bae 3, Tae-Ho Kim 4, Chang Hyung Lee 5, Ki Won Lee 5, Hogyun Seo 6, Kyung-Jin Kim 6 and Sang-Han Lee 1,7,* 1 School of Food Science & Biotechnology, Graduate School, Kyungpook National University, Daegu 41566, Korea; [email protected] (H.-U.S.); [email protected] (E.-K.Y.); [email protected] (C.-Y.Y.) 2 Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA 70808, USA; [email protected] 3 Korea Research Institute of Chemical Technology, Daejeon 34114, Korea; [email protected] 4 Biomedical Research Institute, Kyungpook National University Hospital, Daegu 41940, Korea; [email protected] 5 Major in Biomodulation, Department of Agricultural Biotechnology and Research Institute for Agriculture and Life Sciences, Seoul National University, Seoul 08826, Korea; [email protected] (C.H.L.); [email protected] (K.W.L.) 6 School of Life Sciences, KNU Creative BioResearch Group, Institute of Microorganisms, Kyungpook National University, Daegu 41566, Korea; [email protected] (H.S.); [email protected] (K.-J.K.) 7 knu BnC, Daegu 41566, Korea * Correspondence: [email protected] Received: 18 October 2019; Accepted: 3 December 2019; Published: 5 December 2019 Abstract: To determine the mechanism of action of the effects of phytoalexins in soybeans, we analyzed α-glucosidase inhibition kinetics using Michaelis–Menten plots and Lineweaver–Burk plots. The results showed that the type of inhibition with glyceollin was competitive, that of genistein was noncompetitive, that of daidzein was uncompetitive, and luteolin showed a mixed mode of action. -

Mechanism of Enzymes Involved in Leukotriene C4 Biosynthesis
From Department of Medical Biochemistry and Biophysics Karolinska Institutet, Stockholm, Sweden MECHANISM OF ENZYMES INVOLVED IN LEUKOTRIENE C4 BIOSYNTHESIS H. R. Shabbir Ahmad Stockholm 2016 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by E-Print AB © H. R. Shabbir Ahmad, 2016 ISBN 978-91-7676-368-1 Mechanism of Enzymes Involved in Leukotriene C4 Biosynthesis THESIS FOR DOCTORAL DEGREE (Ph.D.) By H. R. Shabbir Ahmad Opponent: Principal Supervisor: Tim Mantle, PhD Professor Jesper Z. Haeggström Trinity College Dublin Karolinska Institutet Ireland. Department of Medical Biochemistry and Biophysics Division of Chemistry II Examination Board: Co-supervisor(s): Professor Hans-Erik Claesson Karolinska Institutet Agnes Rinaldo-Matthis, PhD Department of Medicine, Solna. Karolinska Institutet Department of Medical Biochemistry and Professor Pia Ädelroth Biophysics Stockholm University Division of Chemistry II Department of Biochemistry and Biophysics Professor Ralf Morgenstern Docent Ylva Ivarsson Karolinska Institutet Uppsala University Institute of Environmental Medicine Department of Chemistry, BMC To my family ABSTRACT Cysteinyl leukotrienes (cys-LTs) are potent proinflammatory mediators associated with various diseases including asthma and allergic rhinitis. Leukotriene C4 synthase (LTC4S) and microsomal glutathione transferase 2 (MGST2) catalyze conjugation of the epoxide intermediate LTA4 with GSH to form LTC4, the parent compound of the cys-LTs. Both enzymes belong to the Membrane-Associated Proteins in Eicosanoid and Glutathione metabolism (MAPEG) super family of integral membrane proteins involved in the generation of lipid mediators and in the metabolism of xenobiotics. This thesis investigates the catalytic mechanism and regulation of LTC4S and MGST2. MGST2 can also catalyze conjugation of glutathione (GSH) with electrophilic substrates, such as 1-chloro-2,4-dinitrobenzene (CDNB) and also possesses GSH-dependent peroxidase activity. -

IF102-1B – RGGT Inhibition
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Access to Research and Communications Annals Phosphonocarboxylates inhibit the second geranylgeranyl addition by Rab Geranylgeranyl Transferase Rudi A. Baron1*, Richard Tavaré1§, Ana C. Figueiredo1, Katarzyna Błażewska2, Boris A. Kashemirov2, Charles E. McKenna2, Frank H. Ebetino3, Adam Taylor4, Michael J. Rogers4, Fraser P. Coxon4, and Miguel C. Seabra1,5,6¶ 1 Molecular Medicine, National Heart and Lung Institute, Imperial College London, London SW7 2AZ, UK, 2 Department of Chemistry, University of Southern California, Los Angeles, California 90089-0744, 3 Procter & Gamble Pharmaceuticals, Cincinnati, Ohio, USA, 4 Bone & Musculoskeletal Programme, Institute of Medical Sciences, University of Aberdeen, Aberdeen, AB25 2ZD, UK, 5Instituto Gulbenkian de Ciência, 2780-156 Oeiras, Portugal, 6Faculdade de Ciências Médicas, Universidade Nova de Lisboa, 1169-056 Lisboa, Portugal * Present address: Cerenis Therapeutics, 31682 Labège, France § Present address: Kings College London, Division of Imaging sciences, The Rayne Institute, 4th floor, Lambeth Wing, St. Thomas Hospital, London SE1 7EH ¶Address correspondence to Miguel C. Seabra: email: [email protected] Running title: Mechanism of RGGT inhibition by bisphosphonates Rab geranylgeranyl transferase demonstrate directly that in the presence of (RGGT) catalyzes the post-translational (+)-3-IPEHPC, Rab-CC and Rab-CXC geranylgeranyl (GG) modification of (usually) proteins are modified by only a single GG two C-terminal cysteines in Rab GTPases. addition. The presence of (+)-3-IPEHPC Here we studied the mechanism of the Rab resulted in a preference for the Rab N- geranylgeranylation reaction by terminal cysteine to be modified first, bisphosphonate analogs in which one suggesting an order of cysteine phosphonate group is replaced by a geranylgeranylation in RGGT catalysis. -

Thymidylate Synthetase Overproduction in 5-Fluorodeoxyuridine-Resistant Mouse Fibroblasts CINDY ROSSANA, LAKSHMI GOLLAKOTA RAO, and LEE F
MOLECULAR AND CELLULAR BIOLOGY, Sept. 1982, p. 1118-1125 Vol. 2, No. 9 0270-7306/82/091118-08$02.00/0 Copyright 0 1982, American Society for Microbiology Thymidylate Synthetase Overproduction in 5-Fluorodeoxyuridine-Resistant Mouse Fibroblasts CINDY ROSSANA, LAKSHMI GOLLAKOTA RAO, AND LEE F. JOHNSON* Department ofBiochemistry, The Ohio State University, Columbus, Ohio 43210 Received 9 February 1982/Accepted 29 April 1982 We describe the isolation and characterization of a series of 5-fluorodeoxyuri- dine (FdUrd)-resistant mouse 3T6 cell lines that overproduce thymidylate synthe- tase (TS) by up to 50-fold compared with the parental cells. The resistant cells were selected by growing 3T6 cells or a methotrexate-resistant 3T6 cell line (M50L3, isolated previously in our laboratory) in gradua$ increasing concentra- tions of FdUrd. Uridine and cytidine were included in the culture medium to reduce toxicity from metabolic products of FdUrd. Cells that were resistant to the drug by virtue of loss of thymidine kinase activity were eliminated by selection in medium containing hypoxanthine, methotrexate, and thymidine. M5OL3 cells were found to adapt to FdUrd more readily than 3T6 cells. A number of clones were isolated that were able to grow in the presence of 3 ,uM (M50L3 derived) or 0.3 ,uM (3T6 derived) FdUrd. Several were found to overproduce TS by 10 to 50- fold compared with normal 3T6 cells. All were found to have thymidine kinase activity, although the enzyme level was significantly reduced in some clones. The overproduced TS was inactivated by 5-fluorodeoxyuridylic acid at the same concentration as the enzyme from 3T6 cells. -

5-Fluorouracil: Mechanisms of Action and Clinical Strategies
REVIEWS 5-FLUOROURACIL: MECHANISMS OF ACTION AND CLINICAL STRATEGIES Daniel B. Longley, D. Paul Harkin and Patrick G. Johnston 5-Fluorouracil (5-FU) is widely used in the treatment of cancer. Over the past 20 years, increased understanding of the mechanism of action of 5-FU has led to the development of strategies that increase its anticancer activity. Despite these advances, drug resistance remains a significant limitation to the clinical use of 5-FU. Emerging technologies, such as DNA microarray profiling, have the potential to identify novel genes that are involved in mediating resistance to 5-FU. Such target genes might prove to be therapeutically valuable as new targets for chemotherapy, or as predictive biomarkers of response to 5-FU-based chemotherapy. FLUOROPYRIMIDINES Antimetabolite drugs work by inhibiting essential Understanding the mechanisms by which 5-FU Antimetabolite drugs such as biosynthetic processes, or by being incorporated into causes cell death and by which tumours become resistant 5-fluorouracil that are fluorinated macromolecules, such as DNA and RNA, and inhibiting to 5-FU is an essential step towards predicting or over- derivatives of pyrimidines. their normal function. The fluoropyrimidine 5-fluo- coming that resistance. So, what do we know about the IRINOTECAN rouracil (5-FU) does both. FLUOROPYRIMIDINES were devel- mechanism of action of 5-FU and what strategies have An anticancer drug that inhibits oped in the 1950s following the observation that rat been used to enhance its activity? DNA MICROARRAY technol- DNA topoisomerase I. It is used hepatomas used the pyrimidine uracil — one of the ogy has the potential to identify novel genes that have key in the treatment of advanced four bases found in RNA — more rapidly than normal roles in mediating resistance to 5-FU-based chemother- colorectal cancer. -

Thymidylate Synthase and Drug Resistance
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DSpace at VU EuropemJ~lofCanVol. %A, Nos. 74 pp. 1299-1305,1995 Copyright 0 1995 Elsevier Science Ltd Pergamon PrintedinGreatBritnin.AUrightsresenred 095wl049/95s9.5o+o.oo Thymidylate Synthase and Drug Resistance G.J. Peters, C.L. van der Wilt, B. van Triest, G. Codacci-Pisanelli, P.G. Johnston, C.J. van Groeningen and H.M. Pinedo Thymidylate synthase is an important target for both fluorinated pyrimidines and for new folate analogues. Resistance to S-fluorouracii (SFU) can be related to insufficient inhibition of thymidylate synthase. The 5FU- nucleotide FdUMP induces inhibition of thymidylate synthase which is enhanced and retained for longer in the presence of increased folate pools, for which leucovorin is a precursor. In a murine model system, 5FU treatment caused a 4-fold induction of thymidylate synthase levels which may have contributed to resistance. Addition of leucovorin to this treatment prevented this induction and increased the antitumour effect 2-3-fold. In the clinical setting, 5FU administration to patients resulted in approximately 50% inhibition of TS after 48 h. The combination with leucovorin resulted in a more pronounced inhibition after 48 h (approximately 70%). A significant relationship was observed with outcome of treatment; when thymidylate synthase levels were high and inhibition was low, no response was observed. A separate study showed that low thymidylate synthase levels appeared to be an independent prognostic factor for adjuvant therapy. Key words: thymidylate synthase, S-fluorouracil, leucovorin, resistance, folates, antifolates EurJ Cancer, Vol. 31A, Nos 718, pp.