Mechanism of Enzymes Involved in Leukotriene C4 Biosynthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Modeling and Functional Characterization of Two Enzymes of the Cyclooxygenase Pathway in Drosophila Melanogaster
City University of New York (CUNY) CUNY Academic Works All Dissertations, Theses, and Capstone Projects Dissertations, Theses, and Capstone Projects 2-2014 Comparative Modeling and Functional Characterization of Two Enzymes of the Cyclooxygenase Pathway in Drosophila melanogaster Yan Qi Graduate Center, City University of New York How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/gc_etds/94 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Comparative Modeling and Functional Characterization of Two Enzymes of the Cyclooxygenase Pathway in Drosophila melanogaster by Yan Qi A dissertation submitted to the Graduate Faculty in Biology in partial fulfillment of the requirements for the degree of Doctor of Philosophy, The City University of New York 2014 i © 2014 Yan Qi All Rights Reserved ii This manuscript has been read and accepted for the Graduate Faculty in Biology in satisfaction of the dissertation requirement for the degree of Doctor of Philosophy. _______ ______________________ Date Chair of Examining Committee Dr. Shaneen Singh, Brooklyn College _______ ______________________ Date Executive Officer Dr. Laurel A. Eckhardt ______________________ Dr. Peter Lipke, Brooklyn College ______________________ Dr. Shubha Govind, City College ______________________ Dr. Richard Magliozzo, Brooklyn College ______________________ Dr. Evros Vassiliou, Kean University Supervising Committee The City University of New York iii Abstract Comparative Modeling and Functional Characterization of Two Enzymes of the Cyclooxygenase Pathway in Drosophila melanogaster by Yan Qi Mentor: Shaneen Singh Eicosanoids are biologically active molecules oxygenated from twenty carbon polyunsaturated fatty acids. -

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -
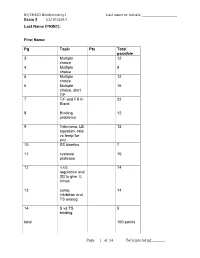
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

GT Lauram.Sc Thesis (FINAL)
OPTIMIZATION OF OVER-EXPRESSION AND PURIFICATION OF HUMAN LEUKOTRIENE C4 SYNTHASE MUTANT R104A FOR STRUCTURE-FUNCTION STUDIES BY TWO-DIMENSIONAL CRYSTALLIZATION AND ELECTRON CRYSTALLOGRAPHY A Thesis Presented to The Academic Faculty by Laura Yaunhee Kim In Partial Fulfillment of the Requirements for the Degree Master of Science in the School of Biology Georgia Institute of Technology December 2012 OPTIMIZATION OF OVER-EXPRESSION AND PURIFICATION OF HUMAN LEUKOTRIENE C4 SYNTHASE MUTANT R104A FOR STRUCTURE-FUNCTION STUDIES BY TWO-DIMENSIONAL CRYSTALLIZATION AND ELECTRON CRYSTALLOGRAPHY Approved by: Dr. Ingeborg Schmidt-Krey, Advisor School of Biology School of Chemistry and Biochemistry Georgia Institute of Technology Dr. Thomas DiChristina School of Biology Georgia Institute of Technology Dr. Raquel L. Lieberman School of Chemistry and Biochemistry Georgia Institute of Technology Date Approved: July 24, 2012 I dedicate this work to my father Sun Dong Kim, mother In Ok Kim, and sister Esther Kim, who have supported me from the very beginning. This work could not have been completed without your love and encouragement. ACKNOWLEDGEMENTS I would like to thank my advisor, Dr. Inga Schmidt-Krey, for guiding and supporting me throughout my academic studies. I would like to thank her for her consistent availability and scientific input during every step of research. Without her guidance this work would not have been possible. I am in debt to my family and friends for their unflinching support and encouragement, without which none of this would be possible. I am also very grateful to all the Schmidt-Krey lab members, especially Matthew Johnson and Maureen Metcalfe, for their support and input into this work. -

Study Protocol
Full title: A Sequential Phase I study of MEK1/2 inhibitors PD-0325901 or Binimetinib combined with cMET inhibitor PF-02341066 in Patients with RAS Mutant and RAS Wild Type (with aberrant c-MET) Colorectal Cancer Short title: MErCuRIC1: MEK and MET Inhibition in Colorectal Cancer Protocol Version & date: Version 9.0; 29Oct2018 Sponsor Protocol Number: OCTO_049 Ethics Number: 14/SC/1010 EudraCT Number: 2014-000463-40 Funding Reference Number 602901-2 Sponsored by the University of Oxford Funder: European Commission’s Seventh Framework Program (FP7) Confidentiality Statement This document contains confidential information that must not be disclosed to anyone other than the Sponsor, the Trials Office, the Investigator Team, host NHS Trust(s), regulatory authorities, and members of the Research Ethics Committee. MErCuRIC1_Protocol_V9.0_29Oct2018 Protocol Template V3.0_18Feb2013 Page 1 of 121 MErCuRIC1 Confidential GENERAL CONTACT INFORMATION Trial Office (OCTO) MErCuRIC Trial Office Oncology Clinical Trials Office (OCTO) Department of Oncology, The University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford Tel: +44 (0)1865 227194 Email: [email protected] Website: http://www.oncology.ox.ac.uk/research/oncology- clinical-trials-office-octo Chief Investigator Professor Mark Middleton Department of Oncology, University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford OX3 7LE Tel: +44 (0)1865 235 315 Email : [email protected] Investigator Professor Richard Wilson and -

9 Glutathione S-Transferases
Enzyme Systems that Metabolise Drugs and Other Xenobiotics. Edited by Costas Ioannides Copyright # 2001 John Wiley & Sons Ltd ISBNs: 0-471-894-66-4 %Hardback); 0-470-84630-5 %Electronic) 9 Glutathione S-transferases Philip J. Sherratt and John D. Hayes University of Dundee, UK Introduction Glutathione S-transferase GST; EC 2.5.1.18) isoenzymes are ubiquitously distributed in nature, being found in organisms as diverse as microbes, insects, plants, ®sh, birds andmammals Hayes andPulford1995). The transferases possess various activities andparticipate in several differenttypes of reaction. Most of these enzymes can catalyse the conjugation of reduced glutathione GSH) with compounds that contain an electrophilic centre through the formation of a thioether bondbetween the sulphur atom of GSH and the substrate Chasseaud 1979; Mannervik 1985). In addition to conjugation reactions, a number of GST isoenzymes exhibit other GSH-dependent catalytic activities including the reduction of organic hydroperoxides Ketterer et al. 1990) andisomerisation of various unsaturatedcompoundsBenson et al. 1977; Jakoby andHabig 1980). These enzymes also have several non-catalytic functions that relate to the sequestering of carcinogens, intracellular transport of a wide spectrum of hydrophobic ligands, and modulation of signal transduction pathways Listowsky 1993; Adler et al. 1999; Cho et al. 2001). Glutathione S-transferases represent a complex grouping of proteins. Two entirely distinct superfamilies of enzyme have evolved that possess transferase activity Hayes andStrange 2000). The ®rst enzymes to be characterisedwere the cytosolic, or soluble, GSTs BoylandandChasseaud1969; Mannervik 1985). To dateat least 16 members of this superfamily have been identi®ed in humans Board et al. 1997, 2000; Hayes and Strange 2000). -

Effects of Synergistic Inhibition on Α-Glucosidase by Phytoalexins In
biomolecules Article Effects of Synergistic Inhibition on α-glucosidase by Phytoalexins in Soybeans Hyeong-U Son 1 , Eun-Kyeong Yoon 1, Chi-Yeol Yoo 1, Chul-Hong Park 2, Myung-Ae Bae 3, Tae-Ho Kim 4, Chang Hyung Lee 5, Ki Won Lee 5, Hogyun Seo 6, Kyung-Jin Kim 6 and Sang-Han Lee 1,7,* 1 School of Food Science & Biotechnology, Graduate School, Kyungpook National University, Daegu 41566, Korea; [email protected] (H.-U.S.); [email protected] (E.-K.Y.); [email protected] (C.-Y.Y.) 2 Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA 70808, USA; [email protected] 3 Korea Research Institute of Chemical Technology, Daejeon 34114, Korea; [email protected] 4 Biomedical Research Institute, Kyungpook National University Hospital, Daegu 41940, Korea; [email protected] 5 Major in Biomodulation, Department of Agricultural Biotechnology and Research Institute for Agriculture and Life Sciences, Seoul National University, Seoul 08826, Korea; [email protected] (C.H.L.); [email protected] (K.W.L.) 6 School of Life Sciences, KNU Creative BioResearch Group, Institute of Microorganisms, Kyungpook National University, Daegu 41566, Korea; [email protected] (H.S.); [email protected] (K.-J.K.) 7 knu BnC, Daegu 41566, Korea * Correspondence: [email protected] Received: 18 October 2019; Accepted: 3 December 2019; Published: 5 December 2019 Abstract: To determine the mechanism of action of the effects of phytoalexins in soybeans, we analyzed α-glucosidase inhibition kinetics using Michaelis–Menten plots and Lineweaver–Burk plots. The results showed that the type of inhibition with glyceollin was competitive, that of genistein was noncompetitive, that of daidzein was uncompetitive, and luteolin showed a mixed mode of action. -

Crystal Structure of Microsomal Prostaglandin E2 Synthase Provides Insight Into Diversity in the MAPEG Superfamily
Crystal structure of microsomal prostaglandin E2 synthase provides insight into diversity in the MAPEG superfamily Tove Sjögrena,1, Johan Nordb, Margareta Eka, Patrik Johanssona, Gang Liub, and Stefan Geschwindnera,1 aDiscovery Sciences, AstraZeneca R&D Mölndal, S-43183 Mölndal, Sweden; and bDepartment of Neuroscience, AstraZeneca R&D Södertälje, S-151 85 Södertälje, Sweden Edited by R. Michael Garavito, Michigan State University, East Lansing, MI, and accepted by the Editorial Board January 17, 2013 (received for review October 24, 2012) fl Prostaglandin E2 (PGE2) is a key mediator in in ammatory re- with mPGES-1. Leukotriene C4 (LTC4) synthase, 5-lipoxygenase sponse. The main source of inducible PGE2, microsomal PGE2 syn- activating protein (FLAP), MGST2, and MGST3 are more dis- thase-1 (mPGES-1), has emerged as an interesting drug target for tantly related with a sequence identity of 15–30%. Initial struc- treatment of pain. To support inhibitor design, we have deter- tural characterization of the MAPEG family was done using mined the crystal structure of human mPGES-1 to 1.2 Å resolution. electron crystallography and showed that the members contain The structure reveals three well-defined active site cavities within four transmembrane helices and are organized as trimers (8, 9). the membrane-spanning region in each monomer interface of This was later confirmed by the X-ray crystal structures of FLAP the trimeric structure. An important determinant of the active (10) and LTC4 synthase (11, 12). There are also low-resolution site cavity is a small cytosolic domain inserted between trans- 3D electron crystallography structures of MGST1 and mPGES-1 membrane helices I and II. -

Structural and Functional Insights of Human 5-Lipoxygenase
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2011 Structural and functional insights of human 5-lipoxygenase Nathaniel Carson Gilbert Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Recommended Citation Gilbert, Nathaniel Carson, "Structural and functional insights of human 5-lipoxygenase" (2011). LSU Doctoral Dissertations. 1747. https://digitalcommons.lsu.edu/gradschool_dissertations/1747 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. STRUCTURAL AND FUNCTIONAL INSIGHTS OF HUMAN 5-LIPOXYGENASE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Biological Sciences by Nathaniel Carson Gilbert B.S., Louisiana State University, 2006 May 2011 DEDICATION This work is in part dedicated to my parents, James and Evelyn Gilbert, who have always supported my educational growth and knew the importance of higher learning. Also to my sister Ashley Ussery and her family: Chris, Chloe, and Noah Ussery for supporting me and reminding me what is most important in life. They, along with the rest of my family and family- in-law, for keeping me motivated in the pursuit of academic excellence. I would also like to dedicate this work to Doug and Emily Caldwell and their son Benjamin Caldwell, whose life was too short, for spurring my interest into all things learned. -

Enzymes: the Biological Accelerators
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Review Article Organic & Medicinal Chem IJ Volume 2 Issue 4 - May 2017 Copyright © All rights are reserved by Ravindra K. Rawal DOI: 10.19080/OMCIJ.2016.01.555594 Enzymes: The Biological Accelerators Sundeep Kaur Manjal, Ramandeep Kaur, Rohit Bhatia and Ravindra K. Rawal* Department of Pharmaceutical Chemistry, ISF College of Pharmacy, India Submission: April 06, 2017; Published: May 26,2017 *Corresponding author: Ravindra K. Rawal, Department of Pharmaceutical Chemistry, ISF College of Pharmacy, GT Road Moga, Punjab 142001, India, Tel: ; Email: Abstract Enzymes are the macromolecular biological catalysts which tend to exhibit tremendous biological value for the human society. These are known to accelerate and catalyze the chemical reactions many times faster than ordinary. Generally, they are known to catalyze more than 5,000 types of biochemical reactions. Numerous enzymes are produced inside the human body which tend to play crucial role in the functioning of biological activities. Some of the enzymes are used commercially such as for the synthesis of antibiotics and also used for household purpose like in manufacturing of washing powder. Apart from this, enzymes serve a variety of functions inside the living organisms. Recently pro-drug approach has gained wide popularity, it is mainly utilised in the lead optimization of the drug molecule. In this review we have tried to discuss different aspects of enzymes, enzyme kinetics, enzyme inhibitors, pro-drugs and their utility with suitable examples. Keywords: Enzyme; Serine; Trypsinogen; Heme; Pro-drug Introduction function are determined by four structural features that are - the Enzymes act as catalysts for almost all of the chemical metal core, the metal binding motif, the second sphere residues reactions that occur in all living organisms [1]. -

IF102-1B – RGGT Inhibition
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Access to Research and Communications Annals Phosphonocarboxylates inhibit the second geranylgeranyl addition by Rab Geranylgeranyl Transferase Rudi A. Baron1*, Richard Tavaré1§, Ana C. Figueiredo1, Katarzyna Błażewska2, Boris A. Kashemirov2, Charles E. McKenna2, Frank H. Ebetino3, Adam Taylor4, Michael J. Rogers4, Fraser P. Coxon4, and Miguel C. Seabra1,5,6¶ 1 Molecular Medicine, National Heart and Lung Institute, Imperial College London, London SW7 2AZ, UK, 2 Department of Chemistry, University of Southern California, Los Angeles, California 90089-0744, 3 Procter & Gamble Pharmaceuticals, Cincinnati, Ohio, USA, 4 Bone & Musculoskeletal Programme, Institute of Medical Sciences, University of Aberdeen, Aberdeen, AB25 2ZD, UK, 5Instituto Gulbenkian de Ciência, 2780-156 Oeiras, Portugal, 6Faculdade de Ciências Médicas, Universidade Nova de Lisboa, 1169-056 Lisboa, Portugal * Present address: Cerenis Therapeutics, 31682 Labège, France § Present address: Kings College London, Division of Imaging sciences, The Rayne Institute, 4th floor, Lambeth Wing, St. Thomas Hospital, London SE1 7EH ¶Address correspondence to Miguel C. Seabra: email: [email protected] Running title: Mechanism of RGGT inhibition by bisphosphonates Rab geranylgeranyl transferase demonstrate directly that in the presence of (RGGT) catalyzes the post-translational (+)-3-IPEHPC, Rab-CC and Rab-CXC geranylgeranyl (GG) modification of (usually) proteins are modified by only a single GG two C-terminal cysteines in Rab GTPases. addition. The presence of (+)-3-IPEHPC Here we studied the mechanism of the Rab resulted in a preference for the Rab N- geranylgeranylation reaction by terminal cysteine to be modified first, bisphosphonate analogs in which one suggesting an order of cysteine phosphonate group is replaced by a geranylgeranylation in RGGT catalysis.