Phase I Enzyme Inhibition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
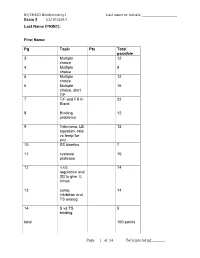
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

The Serpintine Solution
& Experim l e ca n i t in a l l C C f a Journal of Clinical & Experimental o r d l i a o n l o r g u Lucas et al., J Clin Exp Cardiolog 2017, 8:1 y o J Cardiology ISSN: 2155-9880 DOI: 10.4172/2155-9880.1000e150 Editorial Open Access The Serpintine Solution Alexandra Lucas, MD, FRCP(C)1,2,*, Sriram Ambadapadi, PhD1, Brian Mahon, PhD3, Kasinath Viswanathan, PhD4, Hao Chen, MD, PhD5, Liying Liu, MD6, Erbin Dai, MD6, Ganesh Munuswami-Ramanujam, PhD7, Jacek M. Kwiecien, DVM, MSc, PhD8, Jordan R Yaron, PhD1, Purushottam Shivaji Narute, BVSc & AH, MVSc, PhD1,9, Robert McKenna, PhD10, Shahar Keinan, PhD11, Westley Reeves, MD, PhD12, Mark Brantly, MD, PhD13, Carl Pepine, MD, FACC14 and Grant McFadden, PhD1 1Center for Personalized Diagnostics, Biodesign Institute, Arizona State University, Tempe AZ, USA 2Saint Josephs Hospital, Dignity Health Phoenix, Phoenix, AZ, USA 3NIH/ NIDDK, Bethesda MD, USA 4Zydus Research Centre, Ahmedabad, India 5Department of tumor surgery, The Second Hospital of Lanzhou University, Lanzhou, Gansu, P.R.China 6Beth Israel Deaconess Medical Center, Harvard, Boston, MA, USA 7Interdisciplinary Institute of Indian system of Medicine (IIISM), SRM University, Chennai, Tamil Nadu, India 8MacMaster University, Hamilton, ON, Canada 9Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda MD, USA 10Department of Molecular Genetics and Microbiology, University of Florida, Gainesville, FL, USA 11Cloud Pharmaceuticals, Durham, North Carolina, USA 12Division of Rheumatology, University of Florida, -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

Study Protocol
Full title: A Sequential Phase I study of MEK1/2 inhibitors PD-0325901 or Binimetinib combined with cMET inhibitor PF-02341066 in Patients with RAS Mutant and RAS Wild Type (with aberrant c-MET) Colorectal Cancer Short title: MErCuRIC1: MEK and MET Inhibition in Colorectal Cancer Protocol Version & date: Version 9.0; 29Oct2018 Sponsor Protocol Number: OCTO_049 Ethics Number: 14/SC/1010 EudraCT Number: 2014-000463-40 Funding Reference Number 602901-2 Sponsored by the University of Oxford Funder: European Commission’s Seventh Framework Program (FP7) Confidentiality Statement This document contains confidential information that must not be disclosed to anyone other than the Sponsor, the Trials Office, the Investigator Team, host NHS Trust(s), regulatory authorities, and members of the Research Ethics Committee. MErCuRIC1_Protocol_V9.0_29Oct2018 Protocol Template V3.0_18Feb2013 Page 1 of 121 MErCuRIC1 Confidential GENERAL CONTACT INFORMATION Trial Office (OCTO) MErCuRIC Trial Office Oncology Clinical Trials Office (OCTO) Department of Oncology, The University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford Tel: +44 (0)1865 227194 Email: [email protected] Website: http://www.oncology.ox.ac.uk/research/oncology- clinical-trials-office-octo Chief Investigator Professor Mark Middleton Department of Oncology, University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford OX3 7LE Tel: +44 (0)1865 235 315 Email : [email protected] Investigator Professor Richard Wilson and -

Effects of Synergistic Inhibition on Α-Glucosidase by Phytoalexins In
biomolecules Article Effects of Synergistic Inhibition on α-glucosidase by Phytoalexins in Soybeans Hyeong-U Son 1 , Eun-Kyeong Yoon 1, Chi-Yeol Yoo 1, Chul-Hong Park 2, Myung-Ae Bae 3, Tae-Ho Kim 4, Chang Hyung Lee 5, Ki Won Lee 5, Hogyun Seo 6, Kyung-Jin Kim 6 and Sang-Han Lee 1,7,* 1 School of Food Science & Biotechnology, Graduate School, Kyungpook National University, Daegu 41566, Korea; [email protected] (H.-U.S.); [email protected] (E.-K.Y.); [email protected] (C.-Y.Y.) 2 Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA 70808, USA; [email protected] 3 Korea Research Institute of Chemical Technology, Daejeon 34114, Korea; [email protected] 4 Biomedical Research Institute, Kyungpook National University Hospital, Daegu 41940, Korea; [email protected] 5 Major in Biomodulation, Department of Agricultural Biotechnology and Research Institute for Agriculture and Life Sciences, Seoul National University, Seoul 08826, Korea; [email protected] (C.H.L.); [email protected] (K.W.L.) 6 School of Life Sciences, KNU Creative BioResearch Group, Institute of Microorganisms, Kyungpook National University, Daegu 41566, Korea; [email protected] (H.S.); [email protected] (K.-J.K.) 7 knu BnC, Daegu 41566, Korea * Correspondence: [email protected] Received: 18 October 2019; Accepted: 3 December 2019; Published: 5 December 2019 Abstract: To determine the mechanism of action of the effects of phytoalexins in soybeans, we analyzed α-glucosidase inhibition kinetics using Michaelis–Menten plots and Lineweaver–Burk plots. The results showed that the type of inhibition with glyceollin was competitive, that of genistein was noncompetitive, that of daidzein was uncompetitive, and luteolin showed a mixed mode of action. -

Insights Into the Role of Tick Salivary Protease Inhibitors During Ectoparasite–Host Crosstalk
International Journal of Molecular Sciences Review Insights into the Role of Tick Salivary Protease Inhibitors during Ectoparasite–Host Crosstalk Mohamed Amine Jmel 1,† , Hajer Aounallah 2,3,† , Chaima Bensaoud 1, Imen Mekki 1,4, JindˇrichChmelaˇr 4, Fernanda Faria 3 , Youmna M’ghirbi 2 and Michalis Kotsyfakis 1,* 1 Laboratory of Genomics and Proteomics of Disease Vectors, Biology Centre CAS, Institute of Parasitology, Branišovská 1160/31, 37005 Ceskˇ é Budˇejovice,Czech Republic; [email protected] (M.A.J.); [email protected] (C.B.); [email protected] (I.M.) 2 Institut Pasteur de Tunis, Université de Tunis El Manar, LR19IPTX, Service d’Entomologie Médicale, Tunis 1002, Tunisia; [email protected] (H.A.); [email protected] (Y.M.) 3 Innovation and Development Laboratory, Innovation and Development Center, Instituto Butantan, São Paulo 05503-900, Brazil; [email protected] 4 Faculty of Science, University of South Bohemia in Ceskˇ é Budˇejovice, 37005 Ceskˇ é Budˇejovice, Czech Republic; [email protected] * Correspondence: [email protected] † These authors contributed equally. Abstract: Protease inhibitors (PIs) are ubiquitous regulatory proteins present in all kingdoms. They play crucial tasks in controlling biological processes directed by proteases which, if not tightly regulated, can damage the host organism. PIs can be classified according to their targeted proteases or their mechanism of action. The functions of many PIs have now been characterized and are showing clinical relevance for the treatment of human diseases such as arthritis, hepatitis, cancer, AIDS, and cardiovascular diseases, amongst others. Other PIs have potential use in agriculture as insecticides, anti-fungal, and antibacterial agents. -

Mechanism of Enzymes Involved in Leukotriene C4 Biosynthesis
From Department of Medical Biochemistry and Biophysics Karolinska Institutet, Stockholm, Sweden MECHANISM OF ENZYMES INVOLVED IN LEUKOTRIENE C4 BIOSYNTHESIS H. R. Shabbir Ahmad Stockholm 2016 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by E-Print AB © H. R. Shabbir Ahmad, 2016 ISBN 978-91-7676-368-1 Mechanism of Enzymes Involved in Leukotriene C4 Biosynthesis THESIS FOR DOCTORAL DEGREE (Ph.D.) By H. R. Shabbir Ahmad Opponent: Principal Supervisor: Tim Mantle, PhD Professor Jesper Z. Haeggström Trinity College Dublin Karolinska Institutet Ireland. Department of Medical Biochemistry and Biophysics Division of Chemistry II Examination Board: Co-supervisor(s): Professor Hans-Erik Claesson Karolinska Institutet Agnes Rinaldo-Matthis, PhD Department of Medicine, Solna. Karolinska Institutet Department of Medical Biochemistry and Professor Pia Ädelroth Biophysics Stockholm University Division of Chemistry II Department of Biochemistry and Biophysics Professor Ralf Morgenstern Docent Ylva Ivarsson Karolinska Institutet Uppsala University Institute of Environmental Medicine Department of Chemistry, BMC To my family ABSTRACT Cysteinyl leukotrienes (cys-LTs) are potent proinflammatory mediators associated with various diseases including asthma and allergic rhinitis. Leukotriene C4 synthase (LTC4S) and microsomal glutathione transferase 2 (MGST2) catalyze conjugation of the epoxide intermediate LTA4 with GSH to form LTC4, the parent compound of the cys-LTs. Both enzymes belong to the Membrane-Associated Proteins in Eicosanoid and Glutathione metabolism (MAPEG) super family of integral membrane proteins involved in the generation of lipid mediators and in the metabolism of xenobiotics. This thesis investigates the catalytic mechanism and regulation of LTC4S and MGST2. MGST2 can also catalyze conjugation of glutathione (GSH) with electrophilic substrates, such as 1-chloro-2,4-dinitrobenzene (CDNB) and also possesses GSH-dependent peroxidase activity. -

Enzymes: the Biological Accelerators
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Review Article Organic & Medicinal Chem IJ Volume 2 Issue 4 - May 2017 Copyright © All rights are reserved by Ravindra K. Rawal DOI: 10.19080/OMCIJ.2016.01.555594 Enzymes: The Biological Accelerators Sundeep Kaur Manjal, Ramandeep Kaur, Rohit Bhatia and Ravindra K. Rawal* Department of Pharmaceutical Chemistry, ISF College of Pharmacy, India Submission: April 06, 2017; Published: May 26,2017 *Corresponding author: Ravindra K. Rawal, Department of Pharmaceutical Chemistry, ISF College of Pharmacy, GT Road Moga, Punjab 142001, India, Tel: ; Email: Abstract Enzymes are the macromolecular biological catalysts which tend to exhibit tremendous biological value for the human society. These are known to accelerate and catalyze the chemical reactions many times faster than ordinary. Generally, they are known to catalyze more than 5,000 types of biochemical reactions. Numerous enzymes are produced inside the human body which tend to play crucial role in the functioning of biological activities. Some of the enzymes are used commercially such as for the synthesis of antibiotics and also used for household purpose like in manufacturing of washing powder. Apart from this, enzymes serve a variety of functions inside the living organisms. Recently pro-drug approach has gained wide popularity, it is mainly utilised in the lead optimization of the drug molecule. In this review we have tried to discuss different aspects of enzymes, enzyme kinetics, enzyme inhibitors, pro-drugs and their utility with suitable examples. Keywords: Enzyme; Serine; Trypsinogen; Heme; Pro-drug Introduction function are determined by four structural features that are - the Enzymes act as catalysts for almost all of the chemical metal core, the metal binding motif, the second sphere residues reactions that occur in all living organisms [1]. -

Competitive Inhibition That Barrier, As We Shall See
Chapter 4 Catalysis In living systems, speed is everything. Providing the reaction speeds necessary to support life are the catalysts, mostly in the form of enzymes. Catalysis Introduction Introduction Activation Energy General Mechanisms of Action If there is a magical component to life, an argument can surely be made for it Substrate Binding being catalysis. Thanks to catalysis, reactions that could take hundreds of years Enzyme Flexibility to complete in the “real world,” occur in seconds in the presence of a catalyst. Active Site Chymotrypsin Chemical catalysts, like platinum, speed reactions, but enzymes (which are simply Enzyme Parameters super-catalysts with a twist) put chemical catalysts to shame. To understand VMAX & KCAT enzymatic catalysis, we must first understand energy. In Chapter 2, we noted the KM tendency for processes to move in the direction of lower energy. Chemical Perfect Enzymes Lineweaver-Burk Plots reactions follow this universal trend, but they often have a barrier in place that Enzyme Inhibition must be overcome. The secret to catalytic action is reducing the magnitude of Competitive Inhibition that barrier, as we shall see. No Effect On VMAX Increased KM Non-Competitive Inhibition Uncompetitive Inhibition Suicide Inhibition Control of Enzymes Allosterism Covalent Control of Enzymes Other Controls of Enzymes Ribozymes Enzyme Rate Enhancements 81 Activation Energy Another feature to note about catalyzed reactions is Click HERE, HERE, and HERE for The figure below schematically depicts the energy changes that the reduced energy barrier Kevin’s Catalytic Mechanism occur during the progression of a simple reaction. In the figure, lectures on YouTube (also called the activation the energy differences during the reaction are compared for a energy or free energy of catalyzed (plot on the right) and an uncatalyzed reaction (plot on activation) to reach the transition state of the catalyzed reaction. -

Optimizing Defence, Counter-Defence and Counter-Counter Defence in Parasitic and Trophic Interactions – a Modelling Study
1 Optimizing defence, counter-defence and counter-counter defence in parasitic and trophic interactions – A modelling study Stefan Schuster1*, Jan Ewald1,2, Thomas Dandekar2, Sybille Dühring1 1Dept. of Bioinformatics, Friedrich Schiller University of Jena Ernst-Abbe-Platz 2, 07743 Jena, Germany 2Dept. of Bioinformatics, Biocenter Julius Maximilian University of Würzburg Am Hubland, 97074 Würzburg *Corresponding author, e-mail: [email protected] Abstract In host-pathogen interactions, often the host (attacked organism) defends itself by some toxic compound and the parasite, in turn, responds by producing an enzyme that inactivates that compound. In some cases, the host can respond by producing an inhibitor of that enzyme, which can be considered as a counter-counter defence. An example is provided by cephalosporins, β-lactamases and clavulanic acid (an inhibitor of β-lactamases). Here, we tackle the question under which conditions it pays, during evolution, to establish a counter- counter defence rather than to intensify or widen the defence mechanisms. We establish a mathematical model describing this phenomenon, based on enzyme kinetics for competitive inhibition. We use an objective function based on Haber’s rule, which says that the toxic effect is proportional to the time integral of toxin concentration. The optimal allocation of defence and counter-counter defence can be calculated in an analytical way despite the nonlinearity in the underlying differential equation. The calculation provides a threshold value for the dissociation constant of the inhibitor. Only if the inhibition constant is below that threshold, that is, in the case of strong binding of the inhibitor, it pays to have a counter-counter defence. -

IF102-1B – RGGT Inhibition
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Access to Research and Communications Annals Phosphonocarboxylates inhibit the second geranylgeranyl addition by Rab Geranylgeranyl Transferase Rudi A. Baron1*, Richard Tavaré1§, Ana C. Figueiredo1, Katarzyna Błażewska2, Boris A. Kashemirov2, Charles E. McKenna2, Frank H. Ebetino3, Adam Taylor4, Michael J. Rogers4, Fraser P. Coxon4, and Miguel C. Seabra1,5,6¶ 1 Molecular Medicine, National Heart and Lung Institute, Imperial College London, London SW7 2AZ, UK, 2 Department of Chemistry, University of Southern California, Los Angeles, California 90089-0744, 3 Procter & Gamble Pharmaceuticals, Cincinnati, Ohio, USA, 4 Bone & Musculoskeletal Programme, Institute of Medical Sciences, University of Aberdeen, Aberdeen, AB25 2ZD, UK, 5Instituto Gulbenkian de Ciência, 2780-156 Oeiras, Portugal, 6Faculdade de Ciências Médicas, Universidade Nova de Lisboa, 1169-056 Lisboa, Portugal * Present address: Cerenis Therapeutics, 31682 Labège, France § Present address: Kings College London, Division of Imaging sciences, The Rayne Institute, 4th floor, Lambeth Wing, St. Thomas Hospital, London SE1 7EH ¶Address correspondence to Miguel C. Seabra: email: [email protected] Running title: Mechanism of RGGT inhibition by bisphosphonates Rab geranylgeranyl transferase demonstrate directly that in the presence of (RGGT) catalyzes the post-translational (+)-3-IPEHPC, Rab-CC and Rab-CXC geranylgeranyl (GG) modification of (usually) proteins are modified by only a single GG two C-terminal cysteines in Rab GTPases. addition. The presence of (+)-3-IPEHPC Here we studied the mechanism of the Rab resulted in a preference for the Rab N- geranylgeranylation reaction by terminal cysteine to be modified first, bisphosphonate analogs in which one suggesting an order of cysteine phosphonate group is replaced by a geranylgeranylation in RGGT catalysis.