Enzyme Catalysis: Inhibition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 4. Enzyme Kinetics
Chapter 4. Enzyme Kinetics Enzyme Engineering 4.1 Why is enzyme kinetics important? 1. It provides valuable information for enzyme mechanism 2. It gives an insight into the role of an enzyme under physiological conditions 3. It can help show how the enzyme activity is controlled and regulated 1 4.2 How to obtain enzyme kinetics? Measure the formation of product or disappearance of substrate Mg2+ D-Glucose + ATP D-Glucose-6-phosphate + ADP 1. Discontinuous method Add the enzyme, then stop the reaction at different time by adding acid to the sample Measure the concentration of substrate or product using HPLC or other chromatography 4.2 How to obtain enzyme kinetics? 2. Continuous method Mg2+ D-Glucose + ATP D-Glucose-6-phosphate + ADP NADP+ NADPH D-Glucono-δ-lactone 6-phosphate + ADP NADPH absorb at 340nm, while NADP+ does not If a coupled reaction is used, the second reaction should not be rate-limiting (sufficient amount of the enzyme must be provided) Continuous method is not always available 2 4.2 How to obtain enzyme kinetics? Radioactive or fluorescent-labeling is useful Precautions The substrate, buffers, etc. must be extremely pure Enzyme should be pure Enzyme should be stable as the assay is proceeded * Temp, pH must be maintained carefully Once steady state is achieved, reaction rate must be constant and proportional to the enzyme added Initial rate of reaction should be measured to avoid possible complexity 4.3 How to analyze kinetic data? 4.3.1 One substrate reactions k 1 k2 E + S ES E + P k-1 Equilibrium assumption (1913) Second reaction is slower than first reverse reaction (k-1 >> k2) Vmax[S] V0 = Michaelis-Menten eqn K s +[S] [E][S] Vmax = k2[E]0 K = s [ES] 3 4.3 How to analyze kinetic data? Steady state assumption (1925) [ES] is constant Vmax[S] V0 = Km +[S] Vmax = k2[E]0 k2 + k−1 Km = k1 4.3.1 One substrate reactions Lineweaver-Burk equation 1 K 1 1 = m + v [S] Vmax Vmax Eddie-Hofstee equation v V v = max − [S] Km Km 4 4.3.1 One substrate reactions Significance of the result 1. -

Enzyme Activity and Assays Introductory Article
Enzyme Activity and Assays Introductory article Robert K Scopes, La Trobe University, Bundoora, Victoria, Australia Article Contents . Factors that Affect Enzymatic Analysis Enzyme activity refers to the general catalytic properties of an enzyme, and enzyme assays . Initial Rates and Steady State Turnover are standardized procedures for measuring the amounts of specific enzymes in a sample. Measurement of Enzyme Activity . Measurement of Protein Concentration Factors that Affect Enzymatic Analysis . Methods for Purifying Enzymes . Summary Enzyme activity is measured in vitro under conditions that often do not closely resemble those in vivo. The objective of measuring enzyme activity is normally to determine the exactly what the concentration is (some preparations of amount of enzyme present under defined conditions, so unusual substrates may be impure, or the exact amount that activity can be compared between one sample and present may not be known). This is because the rate another, and between one laboratory and another. The measured varies with substrate concentration more rapidly conditions chosen are usually at the optimum pH, as the substrate concentration decreases, as can be seen in ‘saturating’ substrate concentrations, and at a temperature Figure 1. In most cases an enzyme assay has already been that is convenient to control. In many cases the activity is established, and the substrate concentration, buffers and measured in the opposite direction to that of the enzyme’s other parameters used previously should be used again. natural function. Nevertheless, with a complete study of There are many enzymes which do not obey the simple the parameters that affect enzyme activity it should be Michaelis–Menten formula. -

Revised Glossary for AQA GCSE Biology Student Book
Biology Glossary amino acids small molecules from which proteins are A built abiotic factor physical or non-living conditions amylase a digestive enzyme (carbohydrase) that that affect the distribution of a population in an breaks down starch ecosystem, such as light, temperature, soil pH anaerobic respiration respiration without using absorption the process by which soluble products oxygen of digestion move into the blood from the small intestine antibacterial chemicals chemicals produced by plants as a defence mechanism; the amount abstinence method of contraception whereby the produced will increase if the plant is under attack couple refrains from intercourse, particularly when an egg might be in the oviduct antibiotic e.g. penicillin; medicines that work inside the body to kill bacterial pathogens accommodation ability of the eyes to change focus antibody protein normally present in the body acid rain rain water which is made more acidic by or produced in response to an antigen, which it pollutant gases neutralises, thus producing an immune response active site the place on an enzyme where the antimicrobial resistance (AMR) an increasing substrate molecule binds problem in the twenty-first century whereby active transport in active transport, cells use energy bacteria have evolved to develop resistance against to transport substances through cell membranes antibiotics due to their overuse against a concentration gradient antiretroviral drugs drugs used to treat HIV adaptation features that organisms have to help infections; they -

Opportunities for Catalysis in the 21St Century
Opportunities for Catalysis in The 21st Century A Report from the Basic Energy Sciences Advisory Committee BASIC ENERGY SCIENCES ADVISORY COMMITTEE SUBPANEL WORKSHOP REPORT Opportunities for Catalysis in the 21st Century May 14-16, 2002 Workshop Chair Professor J. M. White University of Texas Writing Group Chair Professor John Bercaw California Institute of Technology This page is intentionally left blank. Contents Executive Summary........................................................................................... v A Grand Challenge....................................................................................................... v The Present Opportunity .............................................................................................. v The Importance of Catalysis Science to DOE.............................................................. vi A Recommendation for Increased Federal Investment in Catalysis Research............. vi I. Introduction................................................................................................ 1 A. Background, Structure, and Organization of the Workshop .................................. 1 B. Recent Advances in Experimental and Theoretical Methods ................................ 1 C. The Grand Challenge ............................................................................................. 2 D. Enabling Approaches for Progress in Catalysis ..................................................... 3 E. Consensus Observations and Recommendations.................................................. -

NADPH-Dependent A-Oxidation of Unsaturated Fatty Acids With
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 6673-6677, August 1992 Biochemistry NADPH-dependent a-oxidation of unsaturated fatty acids with double bonds extending from odd-numbered carbon atoms (5-enoyl-CoA/A3,A2-enoyl-CoA isomerase/A3',52'4-dienoyl-CoA isomerase/2,4-dienoyl-CoA reductase) TOR E. SMELAND*, MOHAMED NADA*, DEAN CUEBASt, AND HORST SCHULZ* *Department of Chemistry, City College of the City University of New York, New York, NY 10031; and tJoined Departments of Chemistry, Manhattan College/College of Mount Saint Vincent, Riverdale, NY 10471 Communicated by Salih J. Wakil, April 13, 1992 ABSTRACT The mitochondrial metabolism of 5-enoyl- a recent observation of Tserng and Jin (5) who reported that CoAs, which are formed during the (3-oxidation of unsaturated the mitochondrial -oxidation of 5-enoyl-CoAs is dependent fatty acids with double bonds extending from odd-numbered on NADPH. Their analysis of metabolites by gas chroma- carbon atoms, was studied with mitochondrial extracts and tography/mass spectrometry led them to propose that the purified enzymes of (3-oxidation. Metabolites were identified double bond of 5-enoyl-CoAs is reduced by NADPH to yield spectrophotometrically and by high performance liquid chro- the corresponding saturated fatty acyl-CoAs, which are then matography. 5-cis-Octenoyl-CoA, a putative metabolite of further degraded by P-oxidation. linolenic acid, was efficiently dehydrogenated by medium- This report addresses the question of how 5-enoyl-CoAs chain acyl-CoA dehydrogenase (EC 1.3.99.3) to 2-trans-5-cis- are chain-shortened by P-oxidation. We demonstrate that octadienoyl-CoA, which was isomerized to 3,5-octadienoyl- 5-enoyl-CoAs, after dehydrogenation to 2,5-dienoyl-CoAs, CoA either by mitochondrial A3,A2-enoyl-CoA isomerase (EC can be isomerized to 2,4-dienoyl-CoAs, which are reduced by 5.3.3.8) or by peroxisomal trifunctional enzyme. -

Catalase Kinetics
Experiment #4: Catalase Kinetics MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry 5.310 Laboratory Chemistry 1 EXPERIMENT #4 A Study of the Kinetics of the Enzyme Catalase and its Reaction 2 3 With H2O2. A Further Study on Protein Assay Quantitation of Catalase I. OVERVIEW OF THE EXPERIMENT In this experiment, the student will investigate the enzyme activity of catalase by studying the decomposition of H2O2 to form water and oxygen. Using an oxygen based pressure sensor the student measures the amount of oxygen produced and then calculates the rate of the enzyme catalyzed reaction under various conditions. The student also completes a Protein Assay on an unknown sample of catalase using the Coomassie® Plus Protein Assay from Pierce to determine the protein concentration of the sample. The correlation between the actual and calculated concentration gives an indication of the experimental skills used in carrying out the experiment. II. OBJECTIVES This is an integrated experiment that includes topics from physical chemistry and biochemistry. It is designed to introduce students to the basics of: • Studying the effects of reaction environment, including temperature, and varying substrate concentration on the rate of an enzyme-catalyzed reaction. • Combining physical chemistry and biochemistry, principles and theory, with the goal of determining various biochemical and biophysical constants for the enzyme catalase. • How to acquire experimental kinetic data for an enzyme catalyzed reaction. • Learning how to perform data manipulation in order to extract out information such as rate constants from experimental kinetic data. • Correct handling of UV-VIS Spectroscopy 1 This experiment was synthesized by John J. -

Estimation of the Overall Kinetic Parameters of Enzyme Inactivation Using an Isoconversional Method D
Estimation of the overall kinetic parameters of enzyme inactivation using an isoconversional method D. Oancea, Alexandrina Stuparu, Madalina Nita, Mihaela Puiu, Adina Raducan To cite this version: D. Oancea, Alexandrina Stuparu, Madalina Nita, Mihaela Puiu, Adina Raducan. Estimation of the overall kinetic parameters of enzyme inactivation using an isoconversional method. Biophysical Chemistry, Elsevier, 2008, 138 (1-2), pp.50. 10.1016/j.bpc.2008.09.003. hal-00501718 HAL Id: hal-00501718 https://hal.archives-ouvertes.fr/hal-00501718 Submitted on 12 Jul 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ÔØ ÅÒÙ×Ö ÔØ Estimation of the overall kinetic parameters of enzyme inactivation using an isoconversional method D. Oancea, Alexandrina Stuparu, Madalina Nita, Mihaela Puiu, Adina Raducan PII: S0301-4622(08)00176-2 DOI: doi: 10.1016/j.bpc.2008.09.003 Reference: BIOCHE 5155 To appear in: Biophysical Chemistry Received date: 17 August 2008 Revised date: 2 September 2008 Accepted date: 3 September 2008 Please cite this article as: D. Oancea, Alexandrina Stuparu, Madalina Nita, Mi- haela Puiu, Adina Raducan, Estimation of the overall kinetic parameters of en- zyme inactivation using an isoconversional method, Biophysical Chemistry (2008), doi: 10.1016/j.bpc.2008.09.003 This is a PDF file of an unedited manuscript that has been accepted for publication. -

Part I Principles of Enzyme Catalysis
j1 Part I Principles of Enzyme Catalysis Enzyme Catalysis in Organic Synthesis, Third Edition. Edited by Karlheinz Drauz, Harald Groger,€ and Oliver May. Ó 2012 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2012 by Wiley-VCH Verlag GmbH & Co. KGaA. j3 1 Introduction – Principles and Historical Landmarks of Enzyme Catalysis in Organic Synthesis Harald Gr€oger and Yasuhisa Asano 1.1 General Remarks Enzyme catalysis in organic synthesis – behind this term stands a technology that today is widely recognized as a first choice opportunity in the preparation of a wide range of chemical compounds. Notably, this is true not only for academic syntheses but also for industrial-scale applications [1]. For numerous molecules the synthetic routes based on enzyme catalysis have turned out to be competitive (and often superior!) compared with classic chemicalaswellaschemocatalyticsynthetic approaches. Thus, enzymatic catalysis is increasingly recognized by organic chemists in both academia and industry as an attractive synthetic tool besides the traditional organic disciplines such as classic synthesis, metal catalysis, and organocatalysis [2]. By means of enzymes a broad range of transformations relevant in organic chemistry can be catalyzed, including, for example, redox reactions, carbon–carbon bond forming reactions, and hydrolytic reactions. Nonetheless, for a long time enzyme catalysis was not realized as a first choice option in organic synthesis. Organic chemists did not use enzymes as catalysts for their envisioned syntheses because of observed (or assumed) disadvantages such as narrow substrate range, limited stability of enzymes under organic reaction conditions, low efficiency when using wild-type strains, and diluted substrate and product solutions, thus leading to non-satisfactory volumetric productivities. -

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -

Chapter 7. "Coenzymes and Vitamins" Reading Assignment
Chapter 7. "Coenzymes and Vitamins" Reading Assignment: pp. 192-202, 207-208, 212-214 Problem Assignment: 3, 4, & 7 I. Introduction Many complex metabolic reactions cannot be carried out using only the chemical mechanisms available to the side-chains of the 20 standard amino acids. To perform these reactions, enzymes must rely on other chemical species known broadly as cofactors that bind to the active site and assist in the reaction mechanism. An enzyme lacking its cofactor is referred to as an apoenzyme whereas the enzyme with its cofactor is referred to as a holoenzyme. Cofactors are subdivided into essential ions and organic molecules known as coenzymes (Fig. 7.1). Essential ions, commonly metal ions, may participate in substrate binding or directly in the catalytic mechanism. Coenzymes typically act as group transfer agents, carrying electrons and chemical groups such as acyl groups, methyl groups, etc., depending on the coenzyme. Many of the coenzymes are derived from vitamins which are essential for metabolism, growth, and development. We will use this chapter to introduce all of the vitamins and coenzymes. In a few cases--NAD+, FAD, coenzyme A--the mechanisms of action will be covered. For the remainder of the water-soluble vitamins, discussion of function will be delayed until we encounter them in metabolism. We also will discuss the biochemistry of the fat-soluble vitamins here. II. Inorganic cation cofactors Many enzymes require metal cations for activity. Metal-activated enzymes require or are stimulated by cations such as K+, Ca2+, or Mg2+. Often the metal ion is not tightly bound and may even be carried into the active site attached to a substrate, as occurs in the case of kinases whose actual substrate is a magnesium-ATP complex. -
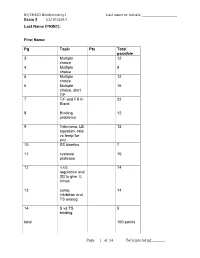
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

Enzymes Main Concepts: •Proteins Are Polymers Made up of Amino Acids
Biology 102 Karen Bledsoe, Instructor Notes http://www.wou.edu/~bledsoek/ Chapter 6, section 4 Topic: Enzymes Main concepts: • Proteins are polymers made up of amino acids. Enzymes are a category of proteins. • Enzymes are catalysts. They speed up the rate of a chemical reaction by reducing the activation energy, which is the energy needed to carry out the reaction. An example of a catalyst: if you put a lit match to a sugar cube, it’s hard to make the sugar catch fire. But if you rub a corner of the sugar cube with ash or charcoal, it catches more easily. The ash or charcoal acts as a catalyst. • Many important chemical reactions in living cells can happen spontaneously, but these reactions happen too rarely and too slowly to sustain life. Enzymes make the reactions happen more quickly and more often. • Some reactions happen too violently to support life. Sugar, when burned, released a lot of energy, but the energy is mostly heat. A rapid reaction like that would overheat our cells. Enzyme-controlled reactions can release energy from sugar in a way that captures most of the energy and loses less of the energy as heat, so that the energy is useful to the cell and does not overheat the cell. • The structure of enzymes allows them to carry out reactions. Each enzyme is shaped to carry out only one specific reaction. This is known as enzyme specificity. Amylase, for example, is an enzyme that breaks down starch into glucose. Amylase only breaks down starch; it does not break down any other molecule, nor does it build starch out of glucose.