Effect of Dietary Flavonoids on Hepatic CYP2E1 and 3A4 Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impaired Hepatic Drug and Steroid Metabolism in Congenital Adrenal
European Journal of Endocrinology (2010) 163 919–924 ISSN 0804-4643 CLINICAL STUDY Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency Dorota Tomalik-Scharte1, Dominique Maiter2, Julia Kirchheiner3, Hannah E Ivison, Uwe Fuhr1 and Wiebke Arlt School of Clinical and Experimental Medicine, Centre for Endocrinology, Diabetes and Metabolism (CEDAM), University of Birmingham, Birmingham B15 2TT, UK, 1Department of Pharmacology, University Hospital, University of Cologne, 50931 Cologne, Germany, 2Department of Endocrinology, University Hospital Saint Luc, 1200 Brussels, Belgium and 3Department of Pharmacology of Natural Products and Clinical Pharmacology, University of Ulm, 89019 Ulm, Germany (Correspondence should be addressed to W Arlt; Email: [email protected]) Abstract Objective: Patients with congenital adrenal hyperplasia due to P450 oxidoreductase (POR) deficiency (ORD) present with disordered sex development and glucocorticoid deficiency. This is due to disruption of electron transfer from mutant POR to microsomal cytochrome P450 (CYP) enzymes that play a key role in glucocorticoid and sex steroid synthesis. POR also transfers electrons to all major drug- metabolizing CYP enzymes, including CYP3A4 that inactivates glucocorticoid and oestrogens. However, whether ORD results in impairment of in vivo drug metabolism has never been studied. Design: We studied an adult patient with ORD due to homozygous POR A287P, the most frequent POR mutation in Caucasians, and her clinically unaffected, heterozygous mother. The patient had received standard dose oestrogen replacement from 17 until 37 years of age when it was stopped after she developed breast cancer. Methods: Both subjects underwent in vivo cocktail phenotyping comprising the oral administration of caffeine, tolbutamide, omeprazole, dextromethorphan hydrobromide and midazolam to assess the five major drug-metabolizing CYP enzymes. -

Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 231 _____________________________ _____________________________ Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21 BY JOHAN BYLUND ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2000 Dissertation for the Degree of Doctor of Philosophy (Faculty of Pharmacy) in Pharmaceutical Pharmacology presented at Uppsala University in 2000 ABSTRACT Bylund, J. 2000. Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid: Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from Faculty of Pharmacy 231 50 pp. Uppsala. ISBN 91-554-4784-8. Cytochrome P450 (P450 or CYP) is an enzyme system involved in the oxygenation of a wide range of endogenous compounds as well as foreign chemicals and drugs. This thesis describes investigations of P450-catalyzed oxygenation of prostaglandins, linoleic and arachidonic acids. The formation of bisallylic hydroxy metabolites of linoleic and arachidonic acids was studied with human recombinant P450s and with human liver microsomes. Several P450 enzymes catalyzed the formation of bisallylic hydroxy metabolites. Inhibition studies and stereochemical analysis of metabolites suggest that the enzyme CYP1A2 may contribute to the biosynthesis of bisallylic hydroxy fatty acid metabolites in adult human liver microsomes. 19R-Hydroxy-PGE and 20-hydroxy-PGE are major components of human and ovine semen, respectively. They are formed in the seminal vesicles, but the mechanism of their biosynthesis is unknown. Reverse transcription-polymerase chain reaction using degenerate primers for mammalian CYP4 family genes, revealed expression of two novel P450 genes in human and ovine seminal vesicles. -

Cholesterol Metabolites 25-Hydroxycholesterol and 25-Hydroxycholesterol 3-Sulfate Are Potent Paired Regulators: from Discovery to Clinical Usage
H OH metabolites OH Review Cholesterol Metabolites 25-Hydroxycholesterol and 25-Hydroxycholesterol 3-Sulfate Are Potent Paired Regulators: From Discovery to Clinical Usage Yaping Wang 1, Xiaobo Li 2 and Shunlin Ren 1,* 1 Department of Internal Medicine, McGuire Veterans Affairs Medical Center, Virginia Commonwealth University, Richmond, VA 23249, USA; [email protected] 2 Department of Physiology and Pathophysiology, School of Basic Medical Sciences, Fudan University, Shanghai 200032, China; [email protected] * Correspondence: [email protected]; Tel.: +1-(804)-675-5000 (ext. 4973) Abstract: Oxysterols have long been believed to be ligands of nuclear receptors such as liver × recep- tor (LXR), and they play an important role in lipid homeostasis and in the immune system, where they are involved in both transcriptional and posttranscriptional mechanisms. However, they are increas- ingly associated with a wide variety of other, sometimes surprising, cell functions. Oxysterols have also been implicated in several diseases such as metabolic syndrome. Oxysterols can be sulfated, and the sulfated oxysterols act in different directions: they decrease lipid biosynthesis, suppress inflammatory responses, and promote cell survival. Our recent reports have shown that oxysterol and oxysterol sulfates are paired epigenetic regulators, agonists, and antagonists of DNA methyl- transferases, indicating that their function of global regulation is through epigenetic modification. In this review, we explore our latest research of 25-hydroxycholesterol and 25-hydroxycholesterol 3-sulfate in a novel regulatory mechanism and evaluate the current evidence for these roles. Citation: Wang, Y.; Li, X.; Ren, S. Keywords: oxysterol sulfates; oxysterol sulfation; epigenetic regulators; 25-hydroxysterol; Cholesterol Metabolites 25-hydroxycholesterol 3-sulfate; 25-hydroxycholesterol 3,25-disulfate 25-Hydroxycholesterol and 25-Hydroxycholesterol 3-Sulfate Are Potent Paired Regulators: From Discovery to Clinical Usage. -

Identification of Novel CYP2E1 Inhibitor to Investigate Cellular and Exosomal CYP2E1-Mediated Toxicity
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 6-2019 Identification of Novel CYP2E1 Inhibitor to Investigate Cellular and Exosomal CYP2E1-Mediated Toxicity Mohammad Arifur Rahman University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Rahman, Mohammad Arifur (0000-0002-5589-0114), "Identification of Novel CYP2E1 Inhibitor to Investigate Cellular and Exosomal CYP2E1-Mediated Toxicity" (2019). Theses and Dissertations (ETD). Paper 482. http://dx.doi.org/10.21007/etd.cghs.2019.0474. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Identification of Novel CYP2E1 Inhibitor to Investigate Cellular and Exosomal CYP2E1-Mediated Toxicity Abstract Cytochrome P450 2E1 (CYP2E1)-mediated hepatic and extra-hepatic toxicity is of significant clinical importance. Diallyl sulfide (DAS) has been shown to prevent xenobiotics such as alcohol- (ALC/ETH), acetaminophen- (APAP) induced toxicity and disease (e.g. HIV-1) pathogenesis. DAS imparts its beneficial effect by inhibiting CYP2E1-mediated metabolism of xenobiotics, especially at high concentration. However, DAS also causes toxicity at relatively high dosages and with long exposure times. The objective of the first project was to find potent ASD analogs which can replace DAS as a research tool or as potential adjuvant therapy in CYP2E1-mediated pathologies. -

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -

Caffeine Metabolism and Cytochrome P450 Enzyme Mrna Expression
Caffeine metabolism and Cytochrome P450 enzyme mRNA expression levels of genetically diverse inbred mouse strains Neal Addicott - CSU East Bay, Michael Malfatti - Lawrence Livermore National Laboratory, Gabriela G. Loots - Lawrence Livermore National Laboratory Metabolic pathways for caffeine 4. Results 1. Introduction (in mice - human overlaps underlined) Metabolites 30 minutes after dose Caffeine is broken down in humans by several enzymes from the Cytochrome Caffeine (1,3,7 - trimethylxanthine) O CH3 (n=6 per strain) CH3 6 N Paraxanthine/Caffeine N 7 Theophylline/Caffeine *Theobromine/Caffeine P450 (CYP) superclass of enzymes. These CYP enzymes are important in Theophylline 1 5 0.06 0.06 0.06 8 (7-N-demethylization) (1,3 - dimethylxanthine) 2 4 9 3 O N 0.05 0.05 0.05 activating or eliminating many medications. The evaluation of caffeine O H N 1,3,7 - trimethyluricacid CH 3 O CH3 N CH eine Peak Area Peak eine eine Peak Area eine Cyp1a2 3 CH 0.04 Area Peak eine 0.04 0.04 f f N f metabolites in a patient has been proposed as a means of estimating the activity 1 7 3 (3-N-demethylization) N Cyp3a4 N1 7 (8-hydrolyzation) 8 OH 0.03 0.03 0.03 of some CYP enzymes, contributing to genetics-based personalized medicine. O 3 N N Cyp1a2 3 O N Paraxanthine (1-N-demethylization) N 0.02 0.02 0.02 CH3 (1,7 - dimethylxanthine) CH3 O CH3 0.01 0.01 0.01 CH3 Theophylline Peak Area / Ca Peak Theophylline Paraxanthine Peak Area / Ca The frequency and distribution of polymorphisms in inbred strains of mice N Area / Ca Peak Theobromine 7 paraxanthine peak area /caffeine peak area /caffeine paraxanthine peak area theophylline peak area /caffeine peak area /caffeine theophylline peak area N1 Theobromine 0 0 peak area /caffeine peak area theobromine 0 0 C57BL/6JC57BL BALB/cJBALB CBA/JCBA/J DBA/2JDBA/2J . -
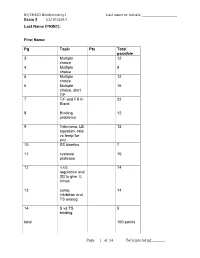
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

TRANSLATIONALLY by AMPK a Dissertation
CHOLESTEROL 7 ALPHA-HYDROXYLASE IS REGULATED POST- TRANSLATIONALLY BY AMPK A dissertation submitted to Kent State University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy By Mauris E.C. Nnamani May 2009 Dissertation written by Mauris E. C. Nnamani B.S, Kent State University, 2006 Ph.D., Kent State University, 2009 Approved by Diane Stroup Advisor Gail Fraizer Members, Doctoral Dissertation Committee S. Vijayaraghavan Arne Gericke Jennifer Marcinkiewicz Accepted by Robert Dorman , Director, School of Biomedical Science John Stalvey , Dean, Collage of Arts and Sciences ii TABLE OF CONTENTS LIST OF FIGURES……………………………………………………………..vi ACKNOWLEDGMENTS……………………………………………………..viii CHAPTER I: INTRODUCTION……………………………………….…........1 a. Bile Acid Synthesis…………………………………………….……….2 i. Importance of Bile Acid Synthesis Pathway………………….….....2 ii. Bile Acid Transport..…………………………………...…...………...3 iii. Bile Acid Synthesis Pathway………………………………………...…4 iv. Classical Bile Acid Synthesis Pathway…..……………………..…..8 Cholesterol 7 -hydroxylase (CYP7A1)……..........………….....8 Transcriptional Regulation of Cholesterol 7 -hydroxylase by Bile Acid-activated FXR…………………………….....…10 CYP7A1 Transcriptional Repression by SHP-dependant Mechanism…………………………………………………...10 CYP7A1 Transcriptional Repression by SHP-independent Mechanism……………………………………..…………….…….11 CYP7A1 Transcriptional Repression by Activated Cellular Kinase…….…………………………...…………………….……12 v. Alternative/ Acidic Bile Acid Synthesis Pathway…………......…….12 Sterol 27-hydroxylase (CYP27A1)……………….…………….12 -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

Potent Inhibition of Human Cytochrome P450 3A Isoforms by Cannabidiol: Role of Phenolic Hydroxyl Groups in the Resorcinol Moiety
Life Sciences 88 (2011) 730–736 Contents lists available at ScienceDirect Life Sciences journal homepage: www.elsevier.com/locate/lifescie Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: Role of phenolic hydroxyl groups in the resorcinol moiety Satoshi Yamaori a, Juri Ebisawa a, Yoshimi Okushima a, Ikuo Yamamoto b, Kazuhito Watanabe a,c,⁎ a Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences, Hokuriku University, Ho-3, Kanagawa-machi, Kanazawa 920-1181, Japan b Department of Hygienic Chemistry, School of Pharmaceutical Sciences, Kyushu University of Health and Welfare, 1714-1 Yoshino-machi, Nobeoka 882-8508, Japan c Organization for Frontier Research in Preventive Pharmaceutical Sciences, Hokuriku University, Ho-3, Kanagawa-machi, Kanazawa 920-1181, Japan article info abstract Article history: Aims: In this study, we examined the inhibitory effects of Δ9-tetrahydrocannabinol (Δ9-THC), cannabidiol Received 4 October 2010 (CBD), and cannabinol (CBN), the three major cannabinoids, on the activity of human cytochrome P450 (CYP) Accepted 10 February 2011 3A enzymes. Furthermore, we investigated the kinetics and structural requirement for the inhibitory effect of CBD on the CYP3A activity. Keywords: Main methods: Diltiazem N-demethylase activity of recombinant CYP3A4, CYP3A5, CYP3A7, and human liver Cannabidiol microsomes (HLMs) in the presence of cannabinoids was determined. Δ9-Tetrahydrocannabinol Key findings: Among the three major cannabinoids, CBD most potently inhibited CYP3A4 and CYP3A5 Cannabinol μ Δ9 CYP3A4 (IC50 =11.7and1.65 M, respectively). The IC50 values of -THC and CBN for CYP3A4 and CYP3A5 were higher μ Δ9 – μ CYP3A5 than 35 M. For CYP3A7, -THC, CBD, and CBN inhibited the activity to a similar extent (IC50 =23 31 M). -

Aripiprazole Therapy and CYP2D6 Genotype
NLM Citation: Dean L. Aripiprazole Therapy and CYP2D6 Genotype. 2016 Sep 22. In: Pratt VM, Scott SA, Pirmohamed M, et al., editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012-. Bookshelf URL: https://www.ncbi.nlm.nih.gov/books/ Aripiprazole Therapy and CYP2D6 Genotype Laura Dean, MD1 Created: September 22, 2016. Introduction Aripiprazole is an atypical antipsychotic used in the management of schizophrenia, bipolar disorder, major depressive disorder, irritability associated with autistic disorder, and treatment of Tourette’s disorder. The metabolism and elimination of aripiprazole is mainly mediated through two enzymes, CYP2D6 and CYP3A4. Approximately 8% of Caucasians, 3–8% of Black/African Americans and up to 2% of Asians cannot metabolize CYP2D6 substrates and are classified as “poor metabolizers” (1). The FDA-approved drug label for aripiprazole states that in CYP2D6 poor metabolizers, half of the usual dose should be administered. In CYP2D6 poor metabolizers who are taking concomitant strong CYP3A4 inhibitors (e.g., itraconazole, clarithromycin), a quarter of the usual dose should be used (Table 1) (2). Table 1. The FDA-recommended dose adjustments for aripiprazole in patients who are known CYP2D6 poor metabolizers and patients taking concomitant CYP2D6 inhibitors, CYP3A4 inhibitors, and/or CYP3A4 inducers (2016) Factors Dosage Adjustments for ABILIFY Known CYP2D6 Poor Metabolizers Administer half of usual dose Known CYP2D6 Poor Metabolizers taking concomitant strong CYP3A4 inhibitors (e.g., Administer a quarter of usual dose itraconazole, clarithromycin) Strong CYP2D6 (e.g., quinidine, fluoxetine, paroxetine) or CYP3A4 inhibitors (e.g., Administer half of usual dose itraconazole, clarithromycin) Strong CYP2D6 and CYP3A4 inhibitors Administer a quarter of usual dose Strong CYP3A4 inducers (e.g., carbamazepine, rifampin) Double usual dose over 1 to 2 weeks Table is adapted from a FDA-approved drug label for aripiprazole (2). -

Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives
pharmaceuticals Article Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives Subrata Deb * , Anthony Allen Reeves and Suki Lafortune Department of Pharmaceutical Sciences, College of Pharmacy, Larkin University, Miami, FL 33169, USA; [email protected] (A.A.R.); [email protected] (S.L.) * Correspondence: [email protected] or [email protected]; Tel.: +1-224-310-7870 or +1-305-760-7479 Received: 9 June 2020; Accepted: 20 July 2020; Published: 23 July 2020 Abstract: Vitamin D3 is an endogenous fat-soluble secosteroid, either biosynthesized in human skin or absorbed from diet and health supplements. Multiple hydroxylation reactions in several tissues including liver and small intestine produce different forms of vitamin D3. Low serum vitamin D levels is a global problem which may origin from differential absorption following supplementation. The objective of the present study was to estimate the physicochemical properties, metabolism, transport and pharmacokinetic behavior of vitamin D3 derivatives following oral ingestion. GastroPlus software, which is an in silico mechanistically-constructed simulation tool, was used to simulate the physicochemical and pharmacokinetic behavior for twelve vitamin D3 derivatives. The Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) Predictor and PKPlus modules were employed to derive the relevant parameters from the structural features of the compounds. The majority of the vitamin D3 derivatives are lipophilic (log P values > 5) with poor water solubility which are reflected in the poor predicted bioavailability. The fraction absorbed values for the vitamin D3 derivatives were low except for calcitroic acid, 1,23S,25-trihydroxy-24-oxo-vitamin D3, and (23S,25R)-1,25-dihydroxyvitamin D3-26,23-lactone each being greater than 90% fraction absorbed.