Enzyme Inhibition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Self-Inhibitory Nature of Metabolic Networks and Its Alleviation Through Compartmentalization
ARTICLE Received 30 Oct 2016 | Accepted 23 May 2017 | Published 10 Jul 2017 DOI: 10.1038/ncomms16018 OPEN The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization Mohammad Tauqeer Alam1,2, Viridiana Olin-Sandoval1,3, Anna Stincone1,w, Markus A. Keller1,4, Aleksej Zelezniak1,5,6, Ben F. Luisi1 & Markus Ralser1,5 Metabolites can inhibit the enzymes that generate them. To explore the general nature of metabolic self-inhibition, we surveyed enzymological data accrued from a century of experimentation and generated a genome-scale enzyme-inhibition network. Enzyme inhibition is often driven by essential metabolites, affects the majority of biochemical processes, and is executed by a structured network whose topological organization is reflecting chemical similarities that exist between metabolites. Most inhibitory interactions are competitive, emerge in the close neighbourhood of the inhibited enzymes, and result from structural similarities between substrate and inhibitors. Structural constraints also explain one-third of allosteric inhibitors, a finding rationalized by crystallographic analysis of allosterically inhibited L-lactate dehydrogenase. Our findings suggest that the primary cause of metabolic enzyme inhibition is not the evolution of regulatory metabolite–enzyme interactions, but a finite structural diversity prevalent within the metabolome. In eukaryotes, compartmentalization minimizes inevitable enzyme inhibition and alleviates constraints that self-inhibition places on metabolism. 1 Department of Biochemistry and Cambridge Systems Biology Centre, University of Cambridge, 80 Tennis Court Road, Cambridge CB2 1GA, UK. 2 Division of Biomedical Sciences, Warwick Medical School, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK. 3 Department of Food Science and Technology, Instituto Nacional de Ciencias Me´dicas y Nutricio´n Salvador Zubira´n, Vasco de Quiroga 15, Tlalpan, 14080 Mexico City, Mexico. -

Plant Protease Inhibitors: a Defense Strategy in Plants
Biotechnology and Molecular Biology Review Vol. 2 (3), pp. 068-085, August 2007 Available online at http://www.academicjournals.org/BMBR ISSN 1538-2273 © 2007 Academic Journals Standard Review Plant protease inhibitors: a defense strategy in plants Huma Habib and Khalid Majid Fazili* Department of Biotechnology, The University of Kashmir, P/O Naseembagh, Hazratbal, Srinagar -190006, Jammu and Kashmir, India. Accepted 7 July, 2007 Proteases, though essentially indispensable to the maintenance and survival of their host organisms, can be potentially damaging when overexpressed or present in higher concentrations, and their activities need to be correctly regulated. An important means of regulation involves modulation of their activities through interaction with substances, mostly proteins, called protease inhibitors. Some insects and many of the phytopathogenic microorganisms secrete extracellular enzymes and, in particular, enzymes causing proteolytic digestion of proteins, which play important roles in pathogenesis. Plants, however, have also developed mechanisms to fight these pathogenic organisms. One important line of defense that plants have to fight these pathogens is through various inhibitors that act against these proteolytic enzymes. These inhibitors are thus active in endogenous as well as exogenous defense systems. Protease inhibitors active against different mechanistic classes of proteases have been classified into different families on the basis of significant sequence similarities and structural relationships. Specific protease inhibitors are currently being overexpressed in certain transgenic plants to protect them against invaders. The current knowledge about plant protease inhibitors, their structure and their role in plant defense is briefly reviewed. Key words: Proteases, enzymes, protease inhibitors, serpins, cystatins, pathogens, defense. Table of content 1. -

Download Download
MM Matin et al. Medical Research Archives vol 8 issue 7. Medical Research Archives RESEARCH ARTICLE 2e`1 PASS Predication, Antiviral, in vitro Antimicrobial, and ADMET Studies of Rhamnopyranoside Esters Authors Mohammed M Matin1, Mohammad HO Roshid2, Sreebash C Bhattacharjee3 and Abul KMS Azad4 Affiliations 1 Bioorganic & Medicinal Chemistry Laboratory, Department of Chemistry, University of Chittagong, Chattogram-4331, Bangladesh 2 Department of Anaesthesia and Intensive Care Medicine, Chattogram Medical College, Chattogram- 4203, Bangladesh 3 Chemical Research Division, Bangladesh Council of Scientific & Industrial Research (BCSIR) Laboratories, Chattogram-4220, Bangladesh 4 Department of Chemistry, Chattogram Govt. College, Chattogram-4203, Bangladesh Corresponding Author: MM Matin E-mail address: [email protected] Tel.: +88 01716 839689. Abstract Sugar derived esters (SEs) with potential antimicrobial activity were found to be a better choice to solve the multidrug resistant (MDR) pathogens due to improved antimicrobial efficacy, biodegradability, non-toxic, and non-allergic properties. In this context, a series of benzyl -L- rhamnopyranoside esters with different chain length (C2-C18) were employed for PASS predication, antiviral, and in vitro antimicrobial activity test. The in vitro antimicrobial tests against four bacterial, and four fungal pathogens along with PASS predication indicated that these sugar esters acted as better antifungals as compared to antibacterial functionality. The study revealed that the incorporation of octanoyl (C8) and lauroyl (C12) group(s) at C-3 position of rhamnopyranoside possessed promising antimicrobial, and anti-carcinogenic potentiality with good pharmacokinetic (pkCSM), and drug likeness properties. Also, attachment of multiple ester groups enhanced various drug likeness, and medicinal chemistry friendliness conditions. Overall, the present findings might be useful for the development of rhamnopyranoside based novel MDR antimicrobial drugs. -

Effect of Statins and ACE Inhibitors Alone and in Combination on Clinical Outcome in Patients with Coronary Heart Disease
Journal of Human Hypertension (2004) 18, 781–788 & 2004 Nature Publishing Group All rights reserved 0950-9240/04 $30.00 www.nature.com/jhh ORIGINAL ARTICLE Effect of statins and ACE inhibitors alone and in combination on clinical outcome in patients with coronary heart disease VG Athyros1, DP Mikhailidis3, AA Papageorgiou2, VI Bouloukos1, AN Pehlivanidis1, AN Symeonidis4 and M Elisaf5, for the GREACE Study Collaborative Group 1Atherosclerosis Unit, Aristotelian University, Hippocration Hospital, Thessaloniki, Greece; 2Department of Clinical Biochemistry, Royal Free Hospital, Royal Free and University College Medical School, Pond Street, London, UK; 32nd Propedeutic Department of Internal Medicine, Aristotelian University, Hippocration Hospital, Thessaloniki, Greece; 4Greek Society of General Practitioners, Thessaloniki, Greece; 5Department of Internal Medicine, Medical School, University of Ioannina, Greece We assessed the ‘synergy’ of statins and angiotensin- point in group A was 31%, (95% CI À48 to À6%, P ¼ 0.01) converting enzyme inhibitors (ACEI) in reducing vascu- in comparison to group B, 59% (95% CI À72 to À48%, lar events in patients with coronary heart disease (CHD). Po0.0001) to group C and 63% (95% CI À74 to À51%, The GREek Atorvastatin and CHD Evaluation (GREACE) Po0.0001) to group D. There was no significant Study, suggested that aggressive reduction of low difference in RRR between groups C and D (9%, CI density lipoprotein cholesterol to 2.59 mmol/l À27–10%, P ¼ 0.1). Other factors (eg the blood pressure) (o100 mg/dl) significantly reduces morbidity and mor- that can influence clinical outcome did not differ tality in CHD patients, in comparison to undertreated significantly between the four treatment groups. -

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -

B-Lactams: Chemical Structure, Mode of Action and Mechanisms of Resistance
b-Lactams: chemical structure, mode of action and mechanisms of resistance Ru´ben Fernandes, Paula Amador and Cristina Prudeˆncio This synopsis summarizes the key chemical and bacteriological characteristics of b-lactams, penicillins, cephalosporins, carbanpenems, monobactams and others. Particular notice is given to first-generation to fifth-generation cephalosporins. This review also summarizes the main resistance mechanism to antibiotics, focusing particular attention to those conferring resistance to broad-spectrum cephalosporins by means of production of emerging cephalosporinases (extended-spectrum b-lactamases and AmpC b-lactamases), target alteration (penicillin-binding proteins from methicillin-resistant Staphylococcus aureus) and membrane transporters that pump b-lactams out of the bacterial cell. Keywords: b-lactams, chemical structure, mechanisms of resistance, mode of action Historical perspective Alexander Fleming first noticed the antibacterial nature of penicillin in 1928. When working with Antimicrobials must be understood as any kind of agent another bacteriological problem, Fleming observed with inhibitory or killing properties to a microorganism. a contaminated culture of Staphylococcus aureus with Antibiotic is a more restrictive term, which implies the the mold Penicillium notatum. Fleming remarkably saw natural source of the antimicrobial agent. Similarly, under- the potential of this unfortunate event. He dis- lying the term chemotherapeutic is the artificial origin of continued the work that he was dealing with and was an antimicrobial agent by chemical synthesis [1]. Initially, able to describe the compound around the mold antibiotics were considered as small molecular weight and isolates it. He named it penicillin and published organic molecules or metabolites used in response of his findings along with some applications of penicillin some microorganisms against others that inhabit the same [4]. -
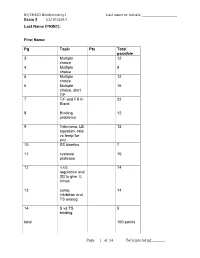
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

The Serpintine Solution
& Experim l e ca n i t in a l l C C f a Journal of Clinical & Experimental o r d l i a o n l o r g u Lucas et al., J Clin Exp Cardiolog 2017, 8:1 y o J Cardiology ISSN: 2155-9880 DOI: 10.4172/2155-9880.1000e150 Editorial Open Access The Serpintine Solution Alexandra Lucas, MD, FRCP(C)1,2,*, Sriram Ambadapadi, PhD1, Brian Mahon, PhD3, Kasinath Viswanathan, PhD4, Hao Chen, MD, PhD5, Liying Liu, MD6, Erbin Dai, MD6, Ganesh Munuswami-Ramanujam, PhD7, Jacek M. Kwiecien, DVM, MSc, PhD8, Jordan R Yaron, PhD1, Purushottam Shivaji Narute, BVSc & AH, MVSc, PhD1,9, Robert McKenna, PhD10, Shahar Keinan, PhD11, Westley Reeves, MD, PhD12, Mark Brantly, MD, PhD13, Carl Pepine, MD, FACC14 and Grant McFadden, PhD1 1Center for Personalized Diagnostics, Biodesign Institute, Arizona State University, Tempe AZ, USA 2Saint Josephs Hospital, Dignity Health Phoenix, Phoenix, AZ, USA 3NIH/ NIDDK, Bethesda MD, USA 4Zydus Research Centre, Ahmedabad, India 5Department of tumor surgery, The Second Hospital of Lanzhou University, Lanzhou, Gansu, P.R.China 6Beth Israel Deaconess Medical Center, Harvard, Boston, MA, USA 7Interdisciplinary Institute of Indian system of Medicine (IIISM), SRM University, Chennai, Tamil Nadu, India 8MacMaster University, Hamilton, ON, Canada 9Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda MD, USA 10Department of Molecular Genetics and Microbiology, University of Florida, Gainesville, FL, USA 11Cloud Pharmaceuticals, Durham, North Carolina, USA 12Division of Rheumatology, University of Florida, -

Challenging the Drug-Likeness Dogma for New Drug Discovery in Tuberculosis
REVIEW published: 03 July 2018 doi: 10.3389/fmicb.2018.01367 Challenging the Drug-Likeness Dogma for New Drug Discovery in Tuberculosis Diana Machado 1, Miriam Girardini 2, Miguel Viveiros 1* and Marco Pieroni 2* 1 Global Health and Tropical Medicine, GHTM, Instituto de Higiene e Medicina Tropical, IHMT, Universidade Nova de Lisboa, UNL, Lisbon, Portugal, 2 P4T Group, Department of Food and Drug, University of Parma, Parma, Italy The emergence of multi- and extensively drug resistant tuberculosis worldwide poses a great threat to human health and highlight the need to discover and develop new, effective and inexpensive antituberculosis agents. High-throughput screening assays Edited by: against well-validated drug targets and structure based drug design have been employed Fernando Rogerio Pavan, to discover new lead compounds. However, the great majority fail to demonstrate any Universidade Estadual Paulista Júlio de Mesquita Filho (UNESP), Brazil antimycobacterial activity when tested against Mycobacterium tuberculosis in whole-cell Reviewed by: screening assays. This is mainly due to some of the intrinsic properties of the bacilli, Pedro Almeida Silva, such as the extremely low permeability of its cell wall, slow growth, drug resistance, Fundação Universidade Federal do drug tolerance, and persistence. In this sense, understanding the pathways involved Rio Grande, Brazil Luiz Augusto Basso, in M. tuberculosis drug tolerance, persistence, and pathogenesis, may reveal new Pontifícia Universidade Católica do Rio approaches for drug development. Moreover, the need for compounds presenting a Grande do Sul, Brazil novel mode of action is of utmost importance due to the emergence of resistance not *Correspondence: Miguel Viveiros only to the currently used antituberculosis agents, but also to those in the pipeline. -

Mechanisms of Action for Small Molecules Revealed by Structural Biology in Drug Discovery
International Journal of Molecular Sciences Review Mechanisms of Action for Small Molecules Revealed by Structural Biology in Drug Discovery Qingxin Li 1,* and CongBao Kang 2,* 1 Guangdong Provincial Engineering Laboratory of Biomass High Value Utilization, Guangdong Provincial Bioengineering Institute (Guangzhou Sugarcane Industry Research Institute), Guangdong Academy of Sciences, Guangzhou 510316, China 2 Experimental Drug Development Centre (EDDC), Agency for Science, Technology and Research (A*STAR), 10 Biopolis Road, Chromos, #05-01, Singapore 138670, Singapore * Correspondence: [email protected] (Q.L.); [email protected] (C.K.); Tel.: +86-020-84168436 (Q.L.); +65-64070602 (C.K.) Received: 12 June 2020; Accepted: 20 July 2020; Published: 24 July 2020 Abstract: Small-molecule drugs are organic compounds affecting molecular pathways by targeting important proteins. These compounds have a low molecular weight, making them penetrate cells easily. Small-molecule drugs can be developed from leads derived from rational drug design or isolated from natural resources. A target-based drug discovery project usually includes target identification, target validation, hit identification, hit to lead and lead optimization. Understanding molecular interactions between small molecules and their targets is critical in drug discovery. Although many biophysical and biochemical methods are able to elucidate molecular interactions of small molecules with their targets, structural biology is the most powerful tool to determine the mechanisms of action for both targets and the developed compounds. Herein, we reviewed the application of structural biology to investigate binding modes of orthosteric and allosteric inhibitors. It is exemplified that structural biology provides a clear view of the binding modes of protease inhibitors and phosphatase inhibitors. -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

Study Protocol
Full title: A Sequential Phase I study of MEK1/2 inhibitors PD-0325901 or Binimetinib combined with cMET inhibitor PF-02341066 in Patients with RAS Mutant and RAS Wild Type (with aberrant c-MET) Colorectal Cancer Short title: MErCuRIC1: MEK and MET Inhibition in Colorectal Cancer Protocol Version & date: Version 9.0; 29Oct2018 Sponsor Protocol Number: OCTO_049 Ethics Number: 14/SC/1010 EudraCT Number: 2014-000463-40 Funding Reference Number 602901-2 Sponsored by the University of Oxford Funder: European Commission’s Seventh Framework Program (FP7) Confidentiality Statement This document contains confidential information that must not be disclosed to anyone other than the Sponsor, the Trials Office, the Investigator Team, host NHS Trust(s), regulatory authorities, and members of the Research Ethics Committee. MErCuRIC1_Protocol_V9.0_29Oct2018 Protocol Template V3.0_18Feb2013 Page 1 of 121 MErCuRIC1 Confidential GENERAL CONTACT INFORMATION Trial Office (OCTO) MErCuRIC Trial Office Oncology Clinical Trials Office (OCTO) Department of Oncology, The University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford Tel: +44 (0)1865 227194 Email: [email protected] Website: http://www.oncology.ox.ac.uk/research/oncology- clinical-trials-office-octo Chief Investigator Professor Mark Middleton Department of Oncology, University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford OX3 7LE Tel: +44 (0)1865 235 315 Email : [email protected] Investigator Professor Richard Wilson and