5 Systematics of High-Pressure Silicate Structures L
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Alkali Pyroxenes and Amphiboles: a Window on Rare
Alkali pyroxenes and amphiboles: a window on rare earth elements and other high field strength elements behavior through the magmatic-hydrothermal transition of peralkaline granitic systems Cyrielle Bernard, Guillaume Estrade, Stefano Salvi, Didier Béziat, Martin Smith To cite this version: Cyrielle Bernard, Guillaume Estrade, Stefano Salvi, Didier Béziat, Martin Smith. Alkali pyroxenes and amphiboles: a window on rare earth elements and other high field strength elements behavior through the magmatic-hydrothermal transition of peralkaline granitic systems. Contributions to Mineralogy and Petrology, Springer Verlag, 2020, 10.1007/s00410-020-01723-y. hal-02989854 HAL Id: hal-02989854 https://hal.archives-ouvertes.fr/hal-02989854 Submitted on 5 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Alkali pyroxenes and amphiboles: a window on Rare Earth Elements and other High Field Strength 2 Elements behavior through the magmatic-hydrothermal transition of peralkaline granitic systems 3 4 Cyrielle Bernarda, Guillaume Estradea⸸, Stefano Salvia, Didier Béziata , Martin Smithb 5 a GET, CNRS, UPS, Université de Toulouse III, Toulouse, France 6 b School of Environment and Technology, University of Brighton, Brighton, BN2 4GJ, UK 7 8 ⸸ Corresponding author: [email protected] 9 ORCID: 0000-0001-6907-7469 10 11 12 Acknowledgments 13 This work was supported by an INSU/TelluS grant from CNRS (French National Center for Scientific 14 Research). -

Ultra-High Pressure Aluminous Titanites in Carbonate-Bearing Eclogites at Shuanghe in Dabieshan, Central China
Ultra-high pressure aluminous titanites in carbonate-bearing eclogites at Shuanghe in Dabieshan, central China D. A. CARSWELL Department of Earth Sciences, University of Sheffield, Sheffield $3 7HF, UK R. N. WILSON Department of Geology, University of Leicester, Leicester LE1 7RH, UK AND M. ZtIAI Institute of Geology, Academia Sinica, P.O. Box 634, Beijing 100029, China Abstract Petrographic features and compositions of titanites in eclogites within the ultra-high pressure metamorphic terrane in central Dabieshan are documented and phase equilibria and thermobarometric implications discussed. Carbonate-bearing eclogite pods in marble at Shuanghe contain primary metamorphic aluminous titanites, with up to 39 mol.% Ca(AI,Fe3+)FSiO4 component. These titanites formed as part of a coesite- bearing eclogite assemblage and thus provide the first direct petrographic evidence that AIFTi_IO_j substitution extends the stability of titanite, relative to futile plus carbonate, to pressures within the coesite stability field. However, it is emphasised that A1 and F contents of such titanites do not provide a simple thermobarometric index of P-T conditions but are constrained by the activity of fluorine, relative to CO2, in metamorphic fluids - as signalled by observations of zoning features in these titanites. These ultra-high pressure titanites show unusual breakdown features developed under more H20-rich amphibolite-facies conditions during exhumation of these rocks. In some samples aluminous titanites have been replaced by ilmenite plus amphibole symplectites, in others by symplectitic intergrowths of secondary, lower AI and F, titanite plus plagioclase. Most other coesite-bearing eclogite samples in the central Dabieshan terrane contain peak assemblage rutile often partly replaced by grain clusters of secondary titanites with customary low AI and F contents. -

Coupled Substitution of H and Minor Elements in Rutile and The
American Mineralogist, Volume 78, pages I 181-1I9I, 1993 Coupled substitution of H and minor elementsin rutile and the implications of high OH contentsin Nb- and Cr-rich rutile from the upper mantle DrvrrrnrosVr-lssopour,os S. S. Papadopulosand Associates,Inc.,7944 Wisconsin Avenue, Bethesda,Maryland 20814-3620,U.S.A. Gponcn R. Rossnn.q,N Division of Geological and Planetary Sciences,California Institute of Technology,Pasadena, California 91125, U.S.A. SrnpHrN E. Hlccrnry Department of Geology, University of Massachusetts,Amherst, Massachusetts01003, U.S.A. Ansrucr Infrared absorption spectraof rutile crystals from a variety of geologicalenvironments (carbonatite,hydrothermal vein, kyanite * rutile + lazulite association,xenoliths that are kimberlite hosted and dominated by Nb- and Cr-rich rutile) exhibit strong absorption in the 3300-cm-' regtrondue to interstitial protons bonded to structure O. In general the proton is located at sites slightly displaced from t/zYz0of the unit cell, although some samplesshow evidenceofadditional protons at tetrahedral interstitial sites.H contents of rutile range up to 0.8 rvto/oHrO, the highest concentrations occurring in mantle-derived Nb- and Cr-rich rutile of metasomatic origin. The role of H in rutile was examined, particularly with respect to its relations to other impurities. Protons are present in the rutile structure to compensatefor trivalent substitutional cations (Cr, Fe, V, Al), which are only partly compensatedby pentavalent ions (Nb, Ta). The possibility of using the H content of rutile as a geohygrometeris illustrated for the caseof coexisting hematite and rutile. INrnonucrroN crystalsto an OH stretch mode. The study by von Hippel (1962) pro- Rutile is known to have a greal affinity for H, and et al. -

Al, Si Retained
Abstracts of Workshop on Transport Properties of the Lower Mantle, Yunishigawa-onsen, Tochigi-ken, Japan, 2008 Scale limits on free-silica seismic scatterers in the lower mantle Craig R. Bina Dept. of Earth and Planetary Sciences, Northwestern University, U.S.A. Seismic velocity anomalies and scatterers of seismic energy in the lower mantle often are attributed to subducted oceanic lithosphere. In particular, silica-saturated basalts in oceanic crust (MORB) under lower mantle conditions should contain high-pressure phases of free silica among assemblages otherwise dominated by silicate perovskite. Free silica phases such as stishovite are expected to generate seismic velocity anomalies that are fast by a few percent relative to surrounding ultramafic peridotite or harzburgite assemblages (Mattern et al. 2002, Bina 2003a, Ricard et al. 2005), and post-stishovite phases such as CaCl2-structured silica may also generate locally slow shear-wave velocity anomalies due to displacive shear-mode transitions (Bina 2003b, Lakshtanov 2007, Konishi et al. 2008). Such models, however, must address the thermodynamic instability of free silica phases in the presence of peridotites or harzburgites, as the silica will react with adjacent ferropericlase (magnesiowüstite) to form silicate perovskite. Thus, any free silica phases preserved in the lower mantle may persist as armored relics, in which silica phases are insulated from surrounding ferropericlase phases by coronas of silicate perovskite. This parallels the situation in crustal metamorphic rocks where, for example, staurolite crystals are often found as armored relics within garnet phases or spinel crystals can be found as relics armored by staurolite poikiloblasts (Whitney 1991, Gil Ibarguchi et al. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

Anorogenic Alkaline Granites from Northeastern Brazil: Major, Trace, and Rare Earth Elements in Magmatic and Metamorphic Biotite and Na-Ma®C Mineralsq
Journal of Asian Earth Sciences 19 (2001) 375±397 www.elsevier.nl/locate/jseaes Anorogenic alkaline granites from northeastern Brazil: major, trace, and rare earth elements in magmatic and metamorphic biotite and Na-ma®c mineralsq J. Pla Cida,*, L.V.S. Nardia, H. ConceicËaÄob, B. Boninc aCurso de PoÂs-GraduacËaÄo em in GeocieÃncias UFRGS. Campus da Agronomia-Inst. de Geoc., Av. Bento GoncËalves, 9500, 91509-900 CEP RS Brazil bCPGG-PPPG/UFBA. Rua Caetano Moura, 123, Instituto de GeocieÃncias-UFBA, CEP- 40210-350, Salvador-BA Brazil cDepartement des Sciences de la Terre, Laboratoire de PeÂtrographie et Volcanologie-Universite Paris-Sud. Centre d'Orsay, Bat. 504, F-91504, Paris, France Accepted 29 August 2000 Abstract The anorogenic, alkaline silica-oversaturated Serra do Meio suite is located within the Riacho do Pontal fold belt, northeast Brazil. This suite, assumed to be Paleoproterozoic in age, encompasses metaluminous and peralkaline granites which have been deformed during the Neoproterozoic collisional event. Preserved late-magmatic to subsolidus amphiboles belong to the riebeckite±arfvedsonite and riebeckite± winchite solid solutions. Riebeckite±winchite is frequently rimmed by Ti±aegirine. Ti-aegirine cores are strongly enriched in Nb, Y, Hf, and REE, which signi®cantly decrease in concentrations towards the rims. REE patterns of Ti-aegirine are strikingly similar to Ti-pyroxenes from the IlõÂmaussaq peralkaline intrusion. Recrystallisation of mineral assemblages was associated with deformation although some original grains are still preserved. Magmatic annite was converted into magnetite and biotite with lower Fe/(Fe 1 Mg) ratios. Recrystallised amphibole is pure riebeckite. Magmatic Ti±Na-bearing pyroxene was converted to low-Ti aegirine 1 titanite ^ astrophyllite/aenigmatite. -
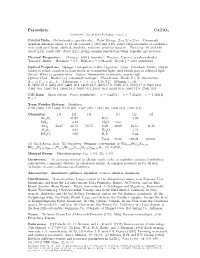
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis
minerals Article Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis Lidia G. Gerasimova 1,2, Anatoly I. Nikolaev 1,2,*, Marina V. Maslova 1,2, Ekaterina S. Shchukina 1,2, Gleb O. Samburov 2, Victor N. Yakovenchuk 1 and Gregory Yu. Ivanyuk 1 1 Nanomaterials Research Centre of Kola Science Centre, Russian Academy of Sciences, 14 Fersman Street, Apatity 184209, Russia; [email protected] (L.G.G.); [email protected] (M.V.M.); [email protected] (E.S.S.); [email protected] (V.N.Y.); [email protected] (G.Y.I.) 2 Tananaev Institute of Chemistry of Kola Science Centre, Russian Academy of Sciences, 26a Fersman Street, Apatity 184209, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-815-557-9231 Received: 4 September 2018; Accepted: 10 October 2018; Published: 12 October 2018 Abstract: Geological setting and mineral composition of (apatite)-nepheline-titanite ore from the Khibiny massif enable selective mining of titanite ore, and its processing with sulfuric-acid method, without preliminary concentration in flotation cells. In this process flow diagram, titanite losses are reduced by an order of magnitude in comparison with a conventional flotation technology. Further, dissolution of titanite in concentrated sulfuric acid produces titanyl sulfate, which, in turn, is a precursor for titanosilicate synthesis. In particular, synthetic analogues of the ivanyukite group minerals, SIV, was synthesized with hydrothermal method from the composition based on titanyl-sulfate, and assayed as a selective cation-exchanger for Cs and Sr. -

Minerals Named After Scientists
Dr. John Andraos, http://www.careerchem.com/NAMED/Minerals.pdf 1 MINERALS NAMED AFTER PEOPLE AND PLACES © Dr. John Andraos, 2003-2011 Department of Chemistry, York University 4700 Keele Street, Toronto, ONTARIO M3J 1P3, CANADA For suggestions, corrections, additional information, and comments please send e-mails to [email protected] http://www.chem.yorku.ca/NAMED/ PEOPLE MINERAL PERSON OR PLACE DESCRIPTION Abelsonite ABELSON, Philip Hauge (1913 - ?) geochemist Abenakiite ABENAKI people, Quebec, Canada Abernathyite ABERNATHY, Jess Mine operator American, b. ? Abswurmbachite ABS-WURMBACH, IRMGARD (1938 - ) mineralogist German, b. ? Adamite ADAM, Gilbert Joseph Zn3(AsO3)2 H2O (1795 - 1881) mineralogist French, b. ? Aegirine AEGIR, Scandinavian god of the sea Afwillite WILLIAMS, Alpheus Fuller (1874 - ?) mine operator DeBeers Consolidated Mines, Kimberley, South Africa Agrellite AGRELL, Stuart O. (? - 1996) mineralogist British, b. ? Agrinierite AGRINIER, Henri (1928 - 1971) mineralogist French, b. ? Aguilarite AGUILAR, P. Superintendent of San Carlos mine, Guanajuato, Mexico Mexican, b. ? Aikenite 2 PbS Cu2S Bi2S5 Andersonite ANDERSON, Dr. John Andraos, http://www.careerchem.com/NAMED/Minerals.pdf 2 Andradite ANDRADA e Silva, Jose B. Ca3Fe2(SiO4)3 de (? - 1838) geologist Brazilian, b. ? Arfvedsonite ARFVEDSON, Johann August (1792 - 1841) Swedish, b. Skagerholms- Bruk, Skaraborgs-Län, Sweden Arrhenite ARRHENIUS, Svante Silico-tantalate of Y, Ce, Zr, (1859 - 1927) Al, Fe, Ca, Be Swedish, b. Wijk, near Uppsala, Sweden Avogardrite AVOGADRO, Lorenzo KBF4, CsBF4 Romano Amedeo Carlo (1776 - 1856) Italian, b. Turin, Italy Babingtonite (Ca, Fe, Mn)SiO3 Fe2(SiO3)3 Becquerelite BECQUEREL, Antoine 4 UO3 7 H2O Henri César (1852 - 1908) French b. Paris, France Berzelianite BERZELIUS, Jöns Jakob Cu2Se (1779 - 1848) Swedish, b. -

Revision #1 the Atomic Arrangement and Electronic Interactions In
This is the peer-reviewed, final accepted version for American Mineralogist, published by the Mineralogical Society of America. The published version is subject to change. Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2021-7851. http://www.minsocam.org/ 1 Revision #1 2 3 The atomic arrangement and electronic interactions in vonsenite at 4 295, 100, and 90 K 5 1, 1, 2 3 4 6 MARC MADERAZZO , JOHN M. HUGHES , M. DARBY DYAR , GEORGE R. ROSSMAN , 5 3 6 7 BRANDON J. ACKLEY , ELIZABETH C. SKLUTE , MARIAN V. LUPULESCU , AND 7 8 JEFFREY CHIARENZELLI 9 10 1Department of Geology, University of Vermont, Burlington, Vermont, 05405, U.S.A. 11 2Department of Geology and Environmental Earth Sciences, Miami University, Oxford, Ohio 45056, U.S.A. 12 3Department of Astronomy, Mount Holyoke College, South Hadley, Massachusetts 01075, U.S.A. 13 4Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125- 14 2500, U.S.A. 15 5Department of Chemistry, University of Vermont, Burlington, Vermont, 05405, U.S.A. 16 6New York State Museum, Research and Collections, 3140 CEC, Albany, New York 12230, U.S.A. 17 7Department of Geology, St. Lawrence University, Canton, New York 13617, U.S.A. 18 19 ABSTRACT 2+ 3+ 20 Vonsenite, Fe 2Fe O2BO3, has been the subject of many studies in the materials-science and 21 condensed-matter-physics communities due to interest in the electronic and magnetic properties 22 and ordering behavior of the phase. One such study, undertaken on synthetic material of 23 endmember composition, reports X-ray diffraction structure refinements that indicate a phase 24 transition from Pbam to Pbnm at or just below approximately 283 K, determined subsequently to 25 arise from a Peierls-like instability. -

The Geochemistry of Gold-Bearing and Gold-Free Pyrite and Marcasite from the Getchell Gold Deposit, Humboldt County, Nevada
UNLV Retrospective Theses & Dissertations 1-1-2001 The geochemistry of gold-bearing and gold-free pyrite and marcasite from the Getchell gold deposit, Humboldt County, Nevada Kelli Diane Weaver University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/rtds Repository Citation Weaver, Kelli Diane, "The geochemistry of gold-bearing and gold-free pyrite and marcasite from the Getchell gold deposit, Humboldt County, Nevada" (2001). UNLV Retrospective Theses & Dissertations. 1344. http://dx.doi.org/10.25669/rwv9-1zgy This Thesis is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Thesis has been accepted for inclusion in UNLV Retrospective Theses & Dissertations by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UM! films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. -

Electrical Conductivity of Minerals and Rocks
1 Electrical conductivity of minerals and rocks Shun-ichiro Karato1 and Duojun Wang1,2 1: Yale University Department of Geology and Geophysics New Haven, CT, USA 2: Key Laboratory of Computational Geodynamics, Graduate University of Chinese Academy of Sciences College of Earth Sciences Beijing 100049, China to be published in “Physics and Chemistry of the Deep Earth” (edited by S. Karato) Wiley-Blackwell 2 SUMMARY Electrical conductivity of most minerals is sensitive to hydrogen (water) content, temperature, major element chemistry and oxygen fugacity. The influence of these parameters on electrical conductivity of major minerals has been characterized for most of the lower crust, upper mantle and transition zone minerals. When the results of properly executed experimental studies are selected, the main features of electrical conductivity in minerals can be interpreted by the physical models of impurity-assisted conduction involving ferric iron and hydrogen-related defects. Systematic trends in hydrogen-related conductivity are found among different types of hydrogen-bearing minerals that are likely caused by the difference in the mobility of hydrogen. A comparison of experimental results with geophysically inferred conductivity shows: (1) Electrical conductivity of the continental lower crust can be explained by a combination of high temperature, high (ferric) iron content presumably associated with dehydration. (2) Electrical conductivity of the asthenosphere can be explained by a modest amount of water (~10-2 wt% in most regions, less than 10-3 wt% in the central/western Pacific). (3) Electrical conductivity of the transition zone requires a higher water content (~10-1 wt% in most regions, ~10-3 wt% in the southern European transition zone, ~1 wt% in the East Asian transition zone).