MYCOTAXON Volume 103, Pp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PLP427R/527R 11-1-05 NAME: QUIZ # 3 1. Described the Common Features of the Organisms Placed in the Deuteromycota, and How
PLP427R/527R 11-1-05 NAME: QUIZ # 3 1. Described the common features of the organisms placed in the Deuteromycota, and how the classes and orders within this phylum are based on form? Explain why this phylum is decreasing in size even though more fungal species are being identified. The organisms in the phylum Deuteromycota are those higher fungi that only have an anamorphic (asexual) stage. They lack a known sexual (teleomorphic) stage. The Deuteromycota is often referred to as a Form-phylum because the organisms are grouped based on form, and may not be the most closely related. As such, groupings are polyphyletic. The classes are defined based on first whether they produce hyphae (Coelomycetes and Hyphomycetes) or are yeast-like (Blastomycetes), and if they do produce hyphae, whether the conidiophores and conidia occur in structures (pycnidia and acervuli) (the Coelomycetes) or not the Hyphomycetes). Orders are based on the type of structure for one class (the Coelomycetes), and on whether or not they produce conidia, or only hyphae for the class lacking asexual spore-bearing structures (the Hyphomycetes). The phylum is decreasing in size primarily because organisms are being re- classified into the Ascomycetes, or some into the Basidiomycetes, based on their molecular phylogenetic relatedness to other species already in those phyla. Some already do not recognize this group as a separate phylum (eg. Kendrick, author of the Fifth Kingdom).. 2. Draw and compare an ascocarp vs. a basidiocarp, included the nuclear content of the hypha forming these sporocarps, name the fertile layer where their respective sexual spores are formed. -

Field Guide to Common Macrofungi in Eastern Forests and Their Ecosystem Functions
United States Department of Field Guide to Agriculture Common Macrofungi Forest Service in Eastern Forests Northern Research Station and Their Ecosystem General Technical Report NRS-79 Functions Michael E. Ostry Neil A. Anderson Joseph G. O’Brien Cover Photos Front: Morel, Morchella esculenta. Photo by Neil A. Anderson, University of Minnesota. Back: Bear’s Head Tooth, Hericium coralloides. Photo by Michael E. Ostry, U.S. Forest Service. The Authors MICHAEL E. OSTRY, research plant pathologist, U.S. Forest Service, Northern Research Station, St. Paul, MN NEIL A. ANDERSON, professor emeritus, University of Minnesota, Department of Plant Pathology, St. Paul, MN JOSEPH G. O’BRIEN, plant pathologist, U.S. Forest Service, Forest Health Protection, St. Paul, MN Manuscript received for publication 23 April 2010 Published by: For additional copies: U.S. FOREST SERVICE U.S. Forest Service 11 CAMPUS BLVD SUITE 200 Publications Distribution NEWTOWN SQUARE PA 19073 359 Main Road Delaware, OH 43015-8640 April 2011 Fax: (740)368-0152 Visit our homepage at: http://www.nrs.fs.fed.us/ CONTENTS Introduction: About this Guide 1 Mushroom Basics 2 Aspen-Birch Ecosystem Mycorrhizal On the ground associated with tree roots Fly Agaric Amanita muscaria 8 Destroying Angel Amanita virosa, A. verna, A. bisporigera 9 The Omnipresent Laccaria Laccaria bicolor 10 Aspen Bolete Leccinum aurantiacum, L. insigne 11 Birch Bolete Leccinum scabrum 12 Saprophytic Litter and Wood Decay On wood Oyster Mushroom Pleurotus populinus (P. ostreatus) 13 Artist’s Conk Ganoderma applanatum -

Mushroom Characterization Part I Illustrated Morphological
Current Research in Environmental & Applied Mycology 8(5): 501–555 (2018) ISSN 2229-2225 www.creamjournal.org Article Doi 10.5943/cream/8/5/3 Mushroom Characterization: Part I – Illustrated Morphological Characteristics Senthilarasu G1, 2* and Kumaresan V3 1 The Energy and Resources Institute, 318, Raheja Arcade, Sector 11, CBD Belapur-400614, Navi Mumbai, Maharashtra, India. 2 Macrofungal Collection of India, 9/174, Gandhi Street, Senneerkuppam, Poonamallee- 600 056, Tamil Nadu, India. 3 Department of Botany, Kanchi Mamunivar Centre for Post Graduate Studies (Autonomous), Puducherry-605008, India. Senthilarasu G, Kumaresan V 2018 – Mushroom Characterization: Part I – Illustrated Morphological Characteristics. Current Research in Environmental & Applied Mycology 8(5), 501–555, Doi 10.5943/cream/8/5/3 Abstract Conventional taxonomy of mushrooms is often not very easy for amateur taxonomists and research scholars to initiate the research on taxonomy and diversity of mushrooms due to the complex morphological characteristics that is often very difficult to comprehend. We illustrate the external morphological characteristics of mushrooms through colorful photographs to facilitate the taxonomic characterization of mushrooms and to promote the research on mushrooms. In addition, a data sheet for morphological characteristics of agaric mushrooms is provided. Key words – agarics – basidiomycetes – fungi – morphology – mushrooms – polypores – taxonomy Introduction It is an endeavor to simplify the morphological characteristics of mushrooms and to make known to the amateur mycologists beyond a shadow of doubt for easy identification of mushrooms. Although, several materials and field guides in the form of drawings are available for illustrating the morphotaxonomy of mushrooms (Largent & Stuntz 1977, Singer 1986, Lodge et al. 2004), still amateur mushroom taxonomists feel it tiresome to take up initial research on mushrooms due to an array of mushroom characteristics to be recorded. -

Sporocarp Ontogeny in Panus (Basidiomycotina): Evolution and Classification
Sporocarp Ontogeny in Panus (Basidiomycotina): Evolution and Classification David S. Hibbett; Shigeyuki Murakami; Akihiko Tsuneda American Journal of Botany, Vol. 80, No. 11. (Nov., 1993), pp. 1336-1348. Stable URL: http://links.jstor.org/sici?sici=0002-9122%28199311%2980%3A11%3C1336%3ASOIP%28E%3E2.0.CO%3B2-M American Journal of Botany is currently published by Botanical Society of America. Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/about/terms.html. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/journals/botsam.html. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. The JSTOR Archive is a trusted digital repository providing for long-term preservation and access to leading academic journals and scholarly literature from around the world. The Archive is supported by libraries, scholarly societies, publishers, and foundations. It is an initiative of JSTOR, a not-for-profit organization with a mission to help the scholarly community take advantage of advances in technology. For more information regarding JSTOR, please contact [email protected]. http://www.jstor.org Tue Jan 8 09:54:21 2008 American Journal of Botany 80(11): 1336-1348. -

Sequestrate Fungi from Patagonian Nothofagus Forests: Cystangium (Russulaceae, Basidiomycota)
Sequestrate fungi from Patagonian Nothofagus forests: Cystangium (Russulaceae, Basidiomycota) Trierveiler-Pereira, L., Smith, M. E., Trappe, J. M., & Nouhra, E. R. (2015). Sequestrate fungi from Patagonian Nothofagus forests: Cystangium (Russulaceae, Basidiomycota). Mycologia, 107(1), 90-103. doi:10.3852/13-302 10.3852/13-302 Allen Press Inc. Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse Mycologia, 107(1), 2015, pp. 90–103. DOI: 10.3852/13-302 # 2015 by The Mycological Society of America, Lawrence, KS 66044-8897 Sequestrate fungi from Patagonian Nothofagus forests: Cystangium (Russulaceae, Basidiomycota) Larissa Trierveiler-Pereira1 Ectomycorrhizal, hypogeous fungi in the Basidio- PPGBOT, Department of Botany, Universidade Federal mycota and Ascomycota are important components of do Rio Grande do Sul, Porto Alegre, Brazil 91501-970 the forest soil environment. Not only do they function Matthew E. Smith as nutrient absorbing organisms for their tree hosts, Department of Plant Pathology, University of Florida, these fungi also improve soil conditions (Perry et al. Gainesville, Florida 32611 1989) and interact with a variety of forest organisms (Trappe and Luoma 1992). In particular, they are an James M. Trappe important food source for animals in ectomycorrhizal Department of Forest Ecosystems and Society, Oregon forests (Maser et al. 1978, Claridge et al. 2002, Vernes State University, Corvallis, Oregon 97331 et al. 2004, Claridge and Trappe 2005, Trappe et al. Eduardo R. Nouhra 2006, Vernes 2010, Katarzˇyte˙ and Kutorga 2011, Instituto Multidisciplinario de Biologı´a Vegetal Schickmann et al. 2012), including those of Argentina (CONICET), Universidad Nacional de Co´rdoba, (Perez Calvo et al. 1989, Nouhra et al. 2005). -

Ultrastructural Studies on the Cultivation Processes and Growth and Development of the Cultivated Mushroom Agaricus Bisporus
Food Structure Volume 4 Number 1 Article 17 1985 Ultrastructural Studies on the Cultivation Processes and Growth and Development of the Cultivated Mushroom Agaricus Bisporus D. A. Wood G. D. Craig P. T. Atkey R. J. Newsam K. Gull Follow this and additional works at: https://digitalcommons.usu.edu/foodmicrostructure Part of the Food Science Commons Recommended Citation Wood, D. A.; Craig, G. D.; Atkey, P. T.; Newsam, R. J.; and Gull, K. (1985) "Ultrastructural Studies on the Cultivation Processes and Growth and Development of the Cultivated Mushroom Agaricus Bisporus," Food Structure: Vol. 4 : No. 1 , Article 17. Available at: https://digitalcommons.usu.edu/foodmicrostructure/vol4/iss1/17 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Food Structure by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. FOOD MICROSTRUCfURE, Vol. 4 (1985). pp. 143-164 0730-5419/85$1 . 00+.05 SEM Inc .. AMF O'Hare (Chicago) , IL 60666-0507 U.S.A. ULTRASTRUCTURAL STUDIES ON THE CULTIVATION PROCESSES AND GROWTH AND DEVELOPMENI' OF THE CULTIVATED MUSHROOM AGARICUS BISPORUS 1 2 1 3 3 D.A. Wood *, G.D. Craig , P. T. Atkey , R.J. Newsarn and K. G.Jll 1 Dept. of Plant Pathology & Microbiology , Glasshouse Crops Research Institute, Littlehampton, 2 West Sussex, BN17 6LP, UK 3 Dept. of Life Sciences, Nottingham Polytechnic, Nottingham, UK Biological Laboratory, University of Kent, canterbury, Kent, UK Abstract Introduction Scanning electron microscopy (SEM) , The production of the cultivated mushroom is transmission electron microscopy and light carried out on a large scale in North America, microscopy have been used t o study various Europe, Australasia and parts of Asia . -

The Protists
The Protists (160,000 living & extinct species; estimates to 200,000 actual species) mostly single-celled, eukaryotes restricted to aquatic, or moist environments: oceans, ponds, lakes, rivers, damp soil, tree bark, snow, etc.; some species are colonial or multicellular; autotrophs & heterotrophs; with or without cell walls; most motile. Genetic analyses have dramatically changed the classification scheme if this group of organisms. In this course we will discuss examples of three main types of protists; algae, protozoa and slime & water molds. Algae [Plant-Like Protists] (22,000 living species) diverse group of mostly unicellular protists, some colonial or multicellular; restricted to aquatic or moist environments: oceans, ponds, lakes, rivers, soil, bark, snow, etc.; photosynthetic with chloroplasts containing chlorophyll and other pigments, most motile; most with cell wall; cell walls of cellulose, proteins,, silica or other materials classified according to their kinds of photosynthetic pigments and composition of cell wall Fire Algae (Dinoflagellates; Phylum Pyrrhophyta, 2100 species) unicellular, many symbiotic as zooxanthellae; some produce cell walls of armored plates, blooms produce toxic red tides, bioluminescent Diatoms (Glass Algae; Phylum Chrysophyta, 28000 living & extinct species) most abundant group of algae; unicellular, radial symmetry, cell walls contain silica; common in freshwater and oceans; source of diatomaceous earth; gliding movement, Euglenas (Phylum Euglenophyta, 1000 species) unicellular, only algae without -

Basidiomycotina
BASIDIOMYCOTINA General Characters • Most advanced group of all fungal classes • Comprises of about 500 genera and 15,000 species • Includes both saprophytic and parasitic species (Mushrooms, Puffballs, Toad stools, Rusts, Smuts etc.) • Parasitic genera spread on stem, leaves, wood or inflorescence (Ustilago, Puccinia, Polyporus, Ganoderma etc.) • Saprophytic species live in decaying wood, logs, dung, dead leaves and humus rich soil (Agaricus, Lycoperdon, Pleurotus, Cyathus, Geastrum etc.) • Characteristics spores are basidiospores, produced on basidia Mycelium • Mycelium is branched, well developed and perennial • Spreading in a fan shaped manner – forming fairy rings in mushrooms • Mycelium spread on the substratum and absorb food • In few genera mycelium form rhizomorphs • Hyphae are septate, septal pore is surrounded by a swollen rim or crescent shaped cap – parenthesome – such septa are called dolipore septum • Not seen in rusts and smuts • Cell wall is made up of chitin • Mycelium occur in three stages • Primary mycelium: Monokaryotic, short lived, formed by germination of basidiospores, represent haplophase, does not bear any sex organs • Secondary mycelium: Dikaryotic, perennial, formed by the fusion (dikaryotisation) of two dissimilar primary mycelium. Represent dikaryotic phase, produce fruiting bodies and show clamp connections • Tertiary mycelium: In higher members, secondary mycelium get organised into specialized tissues forming fruiting bodies. Dikaryotic in nature Clamp Connections • Dikaryotic secondary mycelium grow by -

Pleosporales, Dothideomycetes)
Mycosphere 5 (3): 411–417 (2014) ISSN 2077 7019 www.mycosphere.org Article Mycosphere Copyright © 2014 Online Edition Doi 10.5943/mycosphere/5/3/3 A new species, Lophiostoma versicolor, from Japan (Pleosporales, Dothideomycetes) Hirayama K1, Hashimoto A2, 3 and Tanaka K2 1 Apple Experiment Station, Aomori Prefectural Agriculture and Forestry Research Center, 24 Fukutami, Botandaira, Kuroishi, Aomori 036-0332, Japan 2 Faculty of Agriculture and Life Sciences, Hirosaki University, 3 Bunkyo-cho, Hirosaki, Aomori, 036-8561, Japan 3The United Graduate School of Agricultural Sciences, Iwate University, 18-8 Ueda 3 chome, Morioka 020-8550, Japan Hirayama K, Hashimoto A, Tanaka K 2014 – A new species, Lophiostoma versicolor, from Japan (Pleosporales, Dothideomycetes). Mycosphere 5(3), 411–417, Doi 10.5943/mycosphere/5/3/3 Abstract Lophiostoma versicolor sp. nov. was found on Acer sp. in Japan. This species is characterized by ascomata with a laterally compressed apex; clavate, 2(–4)-spored asci with a long stipe; and verruculose, 3-septate, versicolored ascospores without a sheath or appendages. Phylogenetic analyses based on LSU nrDNA sequences supported the generic placement and species validity of L. versicolor. Key words – ITS – Lophiostomataceae – Lophiotrema – LSU nrDNA – Pleosporomycetidae – Systematics – Taxonomy Introduction During an investigation of bitunicate ascomycetes in Japan, an unidentified fungus was found on dead twigs of Acer sp. The morphological characteristics of the fungus, such as the presence of ascomata with a compressed beak and clavate asci, recall those of Lophiostoma (Hirayama & Tanaka 2011) belonging to the Lophiostomataceae. This fungus, however, is different from any of the existing species of the genus because it possesses 2(–4)-spored asci and verruculose, 3-septate, versicolored ascospores without a sheath or appendages. -
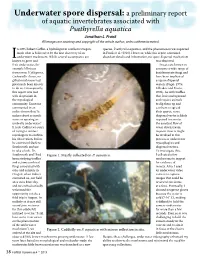
Underwater Spore Dispersal: a Preliminary Report of Aquatic Invertebrates Associated with Psathyrella Aquatica Jonathan L
Underwater spore dispersal: a preliminary report of aquatic invertebrates associated with Psathyrella aquatica Jonathan L. Frank All images are courtesy and copyright of the article author, unless otherwise noted. n 2005 Robert Coffan, a hydrologist in southern Oregon, species, Psathyrella aquatica, and the phenomenon was reported made what is believed to be the first discovery of an in Frank et al. (2010). However, while this report contained underwater mushroom. While several ascomycetes are abundant details and information, no spore dispersal mechanism Iknown to grow and was observed. fruit underwater, for Insects are known to example Vibrissea consume a wide range of truncorum, V. filisporia, basidiomycete fungi and Cudoniella clavus, no have been implicated gilled mushroom had as spore dispersal previously been known vectors (Fogel, 1975; to do so. Consequently, Lilleskov and Bruns, this report was met 2005). As with truffles with skepticism in that fruit underground the mycological and require animals community. Someone to dig them up and commented in an eat them to spread online forum that “it their spores, some makes about as much dispersal vector is likely sense as opening an required to counter umbrella underwater.” the constant flow of It took Coffan two years water downstream. of trying to contact Aquatic insects might mycologists to confirm be involved in this his observation, before process as underwater he convinced Darlene mycophagists and Southworth and me dispersal vectors. to take a look. Dr. To investigate this, Southworth and I had Figure 1. Mayfly collected on P. aquatica. I collected a few been studying truffles mushrooms to inspect and ectomycorrhizal for evidence of fungi associated with insects. -

Membership Just Got a Whole Lot Better. the Website Redesign Is
VOLUME 55: 3 MAY-JUNE 2015 www.namyco.org Membership just got a whole lot better. The website redesign is finally here! As you’ve heard, we contracted with Vieth Consulting this year to makeover the website, install a member management system, and add new member-friendly benefits. In the Members Area behind this basic platform are many new features including forums, calendars, surveys, and ways to communicate news. We designed the NAMA website for you, our members. The member management package includes many perks and more ways to connect to the larger mycological community. For example, instead of Mycophile Editor Dianna Smith laboring over the “send” button for hours, you'll see the newest issue of the newsletter as soon as it’s posted through a web email blast. If you’re curious about the most recent books and field guides to add to your mycological library, you’ll find a section with more than a dozen of the latest book reviews. We’ll be able to let you know about more national and local mycological events through a calendar feature. We will periodically add new stories about our affiliated clubs. We have updated the popular section on mushroom toxins and created a new report form for the Poison Case Registry. Log in to register for the NAMA 2015 Blue Ridge Foray! Our signature annual event, this year hosted by the Asheville Mushroom Club and the Mushroom Club of Georgia, features Alan Bessette as chief mycologist, along with a team of cutting edge scientists and field mycologists. This will be the first year that NAMA has run online registration on our own website (See pages 3-7). -

Common Mushrooms of Indiana State Parks
COMMON Good or Bad? HEALTH HAZARD! Mushrooms have a long history, both in folklore and For every edible mushroom, in the kitchen. Some mushrooms, or “toadstools,” there are lookalikes that may have a dim reputation with humans, but many forest Mushrooms animals eat even the poisonous varieties. Much of a be deadly. Many mushroom mushroom is water, but many have some nutritional of INDIANA STATE PARKS compounds cause allergic value. reactions. Some people develop severe The many different forms result in many uses reactions to mushrooms over time. The three by people. Humans use mushrooms for medicine, mushrooms shown below are poisonous food-and-beverage preparation, biological pest control to humans. There are other types of mush- and dye. rooms not shown here that may be poison- Mushroom? Fungus? ous as well. Mushrooms are just one type of fungus. They are Remember, “when it doubt, throw it out.” non-flowering and can grow in a variety of habitats. Chitin, a support protein usually found in insects and Amanita e other animals, gives fungus its shape. Mushrooms and other fungi reproduce by small dust-like particles called Appearing a ghostly white in spores. Fungi lack chlorophyll, and survive by absorb- color, members of this mushroom ing moisture and nutrients through an underground family are seen alone or in small mass of filaments. “egg” sac groups on the woodland floor. remnant Remnants of the egg-like sac at Can I Hunt Mushrooms at State Park Properties? Mushroom hunting for individual use is permitted the base of the stipe (stalk) on state park properties, and no license is required identify them as members of the to do so.