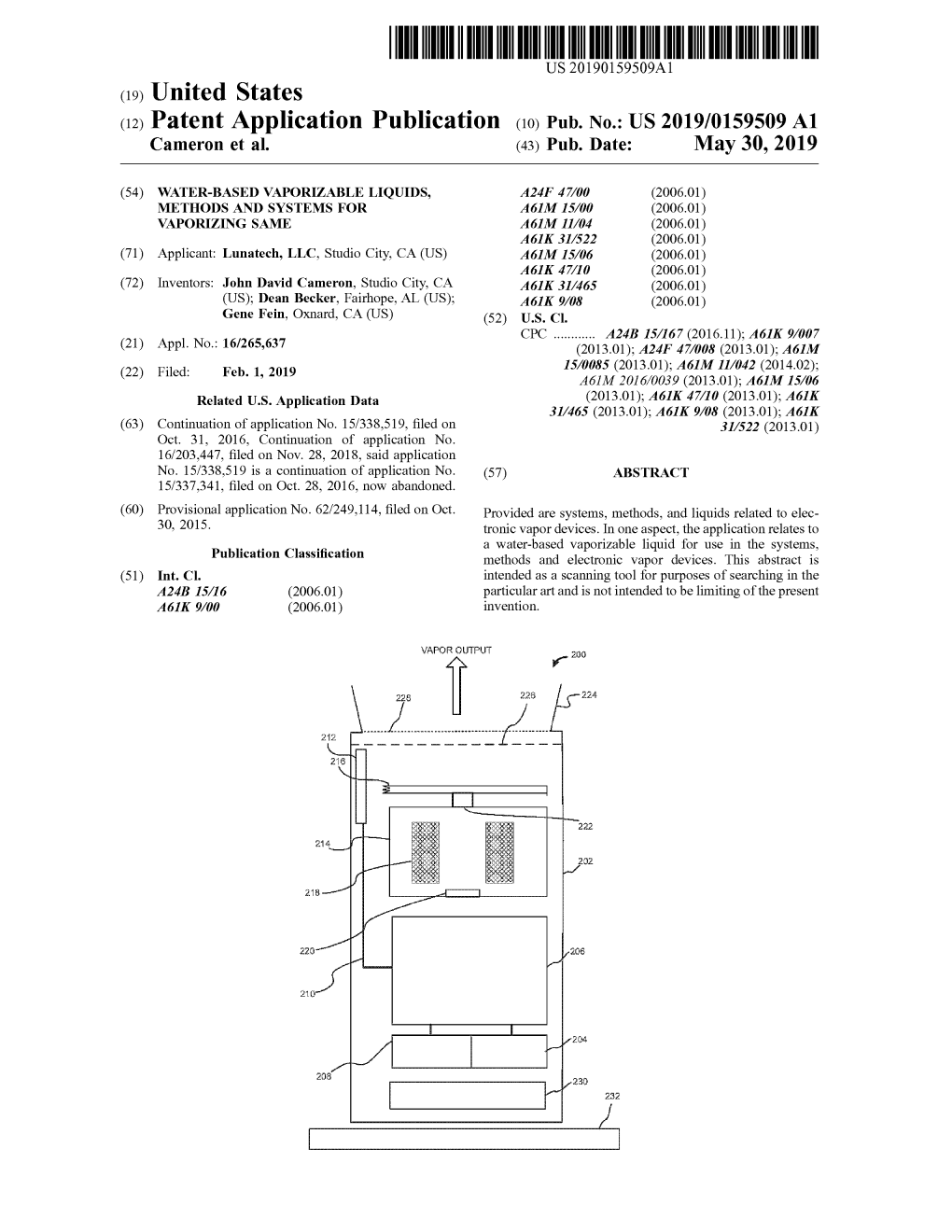
( 12 ) Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0159509 A1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Commission Implementing Regulation (EU)
21.3.2013 EN Official Journal of the European Union L 80/1 II (Non-legislative acts) REGULATIONS COMMISSION IMPLEMENTING REGULATION (EU) No 230/2013 of 14 March 2013 on the withdrawal from the market of certain feed additives belonging to the group of flavouring and appetising substances (Text with EEA relevance) THE EUROPEAN COMMISSION, submitted before that deadline for the only animal category for which those feed additives had been auth orised pursuant to Directive 70/524/EEC. Having regard to the Treaty on the Functioning of the European Union, (4) For transparency purposes, feed additives for which no applications for authorisation were submitted within the Having regard to Regulation (EC) No 1831/2003 of the period specified in Article 10(2) of Regulation (EC) No European Parliament and of the Council of 22 September 1831/2003 were listed in a separate part of the 2003 on additives for use in animal nutrition ( 1 ), and in Community Register of Feed Additives. particular Article 10(5) thereof, (5) Those feed additives should therefore be withdrawn from Whereas: the market as far as their use as flavouring and appetising substances is concerned, except for animal species and categories of animal species for which applications for (1) Regulation (EC) No 1831/2003 provides for the auth authorisation have been submitted. This measure does orisation of additives for use in animal nutrition and not interfere with the use of some of the abovemen for the grounds and procedures for granting such auth tioned additives according to other animal species or orisation. Article 10 of that Regulation provides for the categories of animal species or to other functional re-evaluation of additives authorised pursuant to Council groups for which they may be allowed. -

Test Items for Licensing Examination Krok 1 PHARMACY
MINISTRY OF PUBLIC HEALTH OF UKRAINE Department of human resources policy, education and science Testing Board Student ID Last name Variant ________________ Test items for licensing examination Krok 1 PHARMACY (російськомовний варіант) General Instruction Every one of these numbered questions or unfinished statements in this chapter corresponds to answers or statements endings. Choose the answer (finished statements) that fits best and fill in the circle with the corresponding Latin letter on the answer sheet. Authors of items: Abramov A.V., Aleksandrova K.V., Andronov D.Yu., Bilyk O.V., Blinder O.O., Bobyr V.V., Bobrovska O.A., Bohatyriova O.V., Bodnarchuk O.V., Boieva S.S., Bolokhovska T.O., Bondarenko Yu.I., Bratenko M.K., Buchko O.V., Cherneha H.V., Davydova N.V., Deriuhina L.I., Didenko N.O., Dmytriv A.M., Doroshkevych I.O., Dutka N.M., Dynnyk K.V., Filipova L.O., Havryliuk I.M., Herhel T.M., Hlushkova O.M., Hozhdzinsky S.M., Hrekova T.A., Hrechana O.V., Hruzevsky O.A., Hudyvok Ya.S., Hurmak I.S., Ivanets L.M., Ivanov Ye.I., Kartashova T.V., Kava T.V., Kazakova V.V., Kazmirchuk H.V., Kernychna I.Z., Khlus K.M., Khmelnykova L.I., Klebansky Ye.O., Klopotsky H.A., Klymniuk S.I., Kobylinska L.I., Koldunov V.V., Kolesnyk V.P., Kolesnikova T.O., Komlevoy O.M., Kononenko N.M., Kornijevsky Yu.Y., Kremenska L.V., Krushynska T.Yu., Kryzhanovska A.V., Kryshtal M.V., Kukurychkin Ye.R., Kuznietsova N.L., Kuzmina A.V., Lisnycha A.M., Lychko V.H., Makats Ye.F., Maly V.V., Matvijenko A.H., Menchuk K.M., Minarchenko V.M., Mikheiev A.O., Mishchenko -

56 Subpart F—Flavoring Agents and Related Substances
§ 172.510 21 CFR Ch. I (4–1–12 Edition) needed to produce its intended effect (a) They are used in the minimum but not in excess of 13 parts per million quantity required to produce their in- calculated as anhydrous sodium ferro- tended physical or technical effect and cyanide. in accordance with all the principles of [42 FR 14491, Mar. 15, 1977, as amended at 58 good manufacturing practice. FR 17098, Apr. 1, 1993] (b) In the appropriate forms (plant parts, fluid and solid extracts, con- Subpart F—Flavoring Agents and centrates, absolutes, oils, gums, bal- Related Substances sams, resins, oleoresins, waxes, and dis- tillates) they consist of one or more of § 172.510 Natural flavoring substances the following, used alone or in com- and natural substances used in con- bination with flavoring substances and junction with flavors. adjuvants generally recognized as safe Natural flavoring substances and in food, previously sanctioned for such natural adjuvants may be safely used use, or regulated in any section of this in food in accordance with the fol- part. lowing conditions. Common name Scientific name Limitations Aloe ................................................................ Aloe perryi Baker, A. barbadensis Mill., A. ferox Mill., and hybrids of this sp. with A. africana Mill. and A. spicata Baker. Althea root and flowers .................................. Althea officinalis L. Amyris (West Indian sandalwood) ................. Amyris balsamifera L. Angola weed .................................................. Roccella fuciformis -

RIFM Fragrance Ingredient Safety Assessment, Menthyl Isovalerate CAS Registry Number 16409-46-4
Food and Chemical Toxicology 110 (2017) S486eS495 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Short review RIFM fragrance ingredient safety assessment, menthyl isovalerate CAS Registry Number 16409-46-4 * A.M. Api a, , D. Belsito b, D. Botelho a, D. Browne a, M. Bruze c, G.A. Burton Jr. d, J. Buschmann e, M.L. Dagli f, M. Date a, W. Dekant g, C. Deodhar a, M. Francis a, A.D. Fryer h, K. Joshi a,S.LaCavaa, A. Lapczynski a, D.C. Liebler i, D. O'Brien a, R. Parakhia a,A.Patela, T.M. Penning j, G. Ritacco a, J. Romine a, D. Salvito a, T.W. Schultz k, I.G. Sipes l, Y. Thakkar a, E.H. Theophilus a, A.K. Tiethof a, Y. Tokura m, S. Tsang a, J. Wahler a a Research Institute for Fragrance Materials, Inc., 50 Tice Boulevard, Woodcliff Lake, NJ 07677, USA b Member RIFM Expert Panel, Columbia University Medical Center, Department of Dermatology, 161 Fort Washington Ave., New York, NY 10032, USA c Member RIFM Expert Panel, Malmo University Hospital, Department of Occupational & Environmental Dermatology, Sodra Forstadsgatan 101, Entrance 47, Malmo SE-20502, Sweden d Member RIFM Expert Panel, School of Natural Resources & Environment, University of Michigan, Dana Building G110, 440 Church St., Ann Arbor, MI 58109, USA e Member RIFM Expert Panel, Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Strasse 1, 30625 Hannover, Germany f Member RIFM Expert Panel, University of Sao Paulo, School of Veterinary Medicine and Animal Science, Department of Pathology, Av. -

(12) United States Patent (10) Patent N0.: US 7,265,155 B2 Artman Et A1
US007265155B2 (12) United States Patent (10) Patent N0.: US 7,265,155 B2 Artman et a1. (45) Date of Patent: *Sep. 4, 2007 (54) TREATING A VARIETY OF PATHOLOGICAL W0 98 08498 A 3/1998 CONDITIONS, INCLUDING SPASTICITY WO WO98/08498 3/1998 AND CONVULSIONS, BY EFFECTING A WO WO99/44623 3/1999 MODULATION OF CNS ACTIVITY WITH W0 WO 01/28516 10/2000 ISOVALERAMIDE, ISOVALERIC ACID, OR A RELATED COMPOUND OTHER PUBLICATIONS Schon and Blau, J Neurol Neurosurg Psychiatry, Sep. 1987: (75) Inventors: Linda D. Artman, Salt Lake City, UT 50(9):1148-1152.* (US); Manuel Balandrin, Sandy, UT Dorland’s Medical Dictionary 27th ed. p. 379* (US); Robert L. Smith, Lansdale, PA Pharmacotherapy, A Pathophysiologic Approach, Dipiro et al.2nd (Us) ed. 1991, pp. 1232, 1238).* Drug facts and comparisons 1999 ed. pp. 1595-1597(“Drug (73) Assignee: NBS Pharmaceuticals, Inc., Salt Lake Facts”).* City, UT (US) Pharmacotherapy, A Pathophysiologic Approach, Dipiro et al. 2nd ed. 1991, pp. 1232 & 1238* Notice: Subject to any disclaimer, the term of this Drug Facts and Comparisons. 1999 ed. pp. 1595-1597 (“Drug patent is extended or adjusted under 35 Facts”).* Julius A. Vida “Advances in Anticonvulsant Drug Development”; USC 154(b) by 0 days. Anticonvulsants (1997) pp. 1-9; Academic Press. Julius A. Vida “Noncyclic Anticonvulsants”; Anticonvulsants This patent is subject to a terminal dis (1977) pp. 577-619; Academic Press. claimer. Salim Hadad, et al. “Pharmacokinetic Analysis and Antiepileptic Activity of N-Valproyl Deriatives of GABA and Glycine”; Phar (21) Appl. N0.: 10/614,344 maceutical Research (1995), pp. 905-907; Plenum Publishing Cor poration. -

Cigarette Additives, Carcinogens and Chemicals Nicotine
Cigarette Additives, Carcinogens and Chemicals Nicotine A Destructive Natural Pesticide Which ... Is extremely addictive when smoked Is extremely addictive when chewed Causes addiction as permanent as Is harder to quit than heroin or cocaine alcoholism Is not medicine and its use not therapy Is ineffective as a stand-alone quitting aid Prevents pre-cancerous cells from dying Accelerates cancer tumor growth rates Contributes to artery hardening Has a metabolite which may cause cancer May kill brain cells and impair memory Is linked to lung cancer Likely causes brain damage and Is also a fetus destroying teratogen depression Kills half of adult smokers 13-14 years Is beat by never taking another puff or early chew! 81 Cancer Causing Chemicals Have So Far Been Identified in Cigarettes Acetaldehyde Acetamide Acrylamide Acrylonitrile 2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline (MeIQ) 3-Amino-1,4-dimethyl-5H-pyrido [4,3-b]indole (Trp-P-1) 2-Amino-l-methyl-6-phenyl-1H-imidazo [4,5-b]pyridine (PhlP) 2-Amino-6-methyldipyrido[1,2-a:3',2'-d]imidazole (Glu-P-1) 3-Amino-l-methyl-5H-pyrido {4,3-b]indole (Trp-P-2 2-Amino-3-methyl-9H-pyrido[2,3-b]indole (MeAaC) 2-Amino-9H-pyrido[2,3-b]indole (AaC) 4-Aminobiphenyl 2-Aminodipyrido[1,2-a:3',2'-d]imidazole (Glu-P-2) 0-Anisidine Arsenic Benz[a]anthracene Benzene Benzo[a]pyrene Benzo[b]fluoranthene Benzo[j]fluoranthene Benzo[k]fluoranthene Benzo[b]furan Beryllium 1,3-Butadiene Cadmium Catechol (1,2-benzenediol) p-Chloroaniline Chloroform Cobalt p,p'-DDT Dibenz[a,h]acridine Dibenz[a,j]acridine Dibenz(a,h)anthracene -

Cover Next Page > Cover Next Page >
cover next page > Cover title: The Psychopharmacology of Herbal Medicine : Plant Drugs That Alter Mind, Brain, and Behavior author: Spinella, Marcello. publisher: MIT Press isbn10 | asin: 0262692651 print isbn13: 9780262692656 ebook isbn13: 9780585386645 language: English subject Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. publication date: 2001 lcc: RC483.S65 2001eb ddc: 615/.788 subject: Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. cover next page > < previous page page_i next page > Page i The Psychopharmacology of Herbal Medicine < previous page page_i next page > cover next page > Cover title: The Psychopharmacology of Herbal Medicine : Plant Drugs That Alter Mind, Brain, and Behavior author: Spinella, Marcello. publisher: MIT Press isbn10 | asin: 0262692651 print isbn13: 9780262692656 ebook isbn13: 9780585386645 language: English subject Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. publication date: 2001 lcc: RC483.S65 2001eb ddc: 615/.788 subject: Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. cover next page > < previous page page_ii next page > Page ii This page intentionally left blank. < previous page page_ii next page > < previous page page_iii next page > Page iii The Psychopharmacology of Herbal Medicine Plant Drugs That Alter Mind, Brain, and Behavior Marcello Spinella < previous page page_iii next page > < previous page page_iv next page > Page iv © 2001 Massachusetts Institute of Technology All rights reserved. No part of this book may be reproduced in any form by any electronic or mechanical means (including photocopying, recording, or information storage and retrieval) without permission in writing from the publisher. This book was set in Adobe Sabon in QuarkXPress by Asco Typesetters, Hong Kong and was printed and bound in the United States of America. -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Rhubarb, garden root ..................................... Rheum rhaponticum L ................................................ Do. Rhubarb root .................................................. Rheum officinale Baill., R. palmatum L., or other spp. (excepting R. rhaponticum L.) or hybrids of Rheum grown in China. Roselle ........................................................... Hibiscus sabdariffa L .................................................. Do. Rosin (colophony) .......................................... Pinus palustris Mill., and other Pinus spp .................. Do. St. Johnswort leaves, flowers, and caulis ...... Hypericum perforatum L ............................................. Hypericin-free alcohol dis- tillate form only; in alco- holic beverages only Sandalwood, white (yellow, or East Indian) ... Santalum album L. Sandarac ........................................................ Tetraclinis articulata (Vahl.), Mast .............................. In alcoholic beverages only Sarsaparilla ..................................................... Smilax aristolochiaefolia Mill., (Mexican sarsaparilla), S. regelii Killip et Morton (Honduras sarsaparilla), S. febrifuga Kunth (Ecuadorean sarsaparilla), or undetermined Smilax spp. (Ecuadorean or Central American sarsaparilla). Sassafras leaves ............................................ Sassafras albidum (Nutt.) Nees ................................. Safrole free Senna, Alexandria ......................................... -

7 Príloha Č. 1 K Tretej Hlave Druhej Časti Potravinového Kódexu
Príloha č. 1 k tretej hlave druhej časti potravinového kódexu Kontaminanty v potravinách Časť A Chemické prvky 1. Chemické prvky v potravinách sú prvky, ktoré sa dostávajú do potravín z pôdy, vody a z ovzdušia ako kontaminanty; niektoré z nich môžu byť aj prirodzenou zložkou potravín. Pri posudzovaní ich zisteného množstva v potravinách nie je rozhodujúci spôsob, akým sa do potravín dostali a či sa vyskytujú v čistej forme alebo v zlúčeninách. Na posudzovanie rádionuklidov v potravinách sa vzťahujú osobitné požiadavky podľa šiestej hlavy druhej časti potravinového kódexu. 2. Najvyššie prípustné množstvo chemických prvkov vo výrobkoch na dojčenskú a detskú výživu sa vzťahuje na ich konzumnú formu podľa ich odporúčaného najmenšieho zriedenia. 3. Ak sú ryby určené celé na konzum, najvyššie prípustné množstvo sa vzťahuje na celé ryby. Chemický Najvyššie prípustné Potravina prvok množstvo v mg.kg-1 Arzén 0,05 mlieko, potraviny pre dojčatá a malé deti na báze mlieka 0,1 mäso a mäsové výrobky, múka, vajcia a výrob- ky z vajec, rastlinné tuky a oleje a živočíšne tuky, potraviny pre dojčatá a malé deti na ovocnom a zeleninovom základe 0,2 zverina a výrobky z nej, obilie a výrobky z neho, ocot, nápoje vrátane nápojov z ovocia a zeleniny, výrobky z mlieka okrem tvrdých syrov 0,3 zemiaky 0,5 tvrdé syry, ovocie a zelenina a výrobky z nich, strukoviny, čerstvé huby, čokoláda a výrobky z čo- kolády 1,0 vnútornosti a výrobky z nich, ryby sladkovodné a výrobky z nich, cukor a ostatné prírodné sladidlá okrem práškového cukru, cukrovinky, kakaový prášok -

WO 2017/120012 Al 13 July 20 17 (13.07.2017) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/120012 Al 13 July 20 17 (13.07.2017) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/185 (2006.01) A61K 31/336 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, PCT/US20 16/067024 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, 15 December 2016 (15. 12.2016) KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, (25) Filing Language: English NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, (26) Publication Language: English RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, (30) Priority Data: ZA, ZM, ZW. 62/275,182 5 January 2016 (05.01 .2016) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: THE REGENTS OF THE UNIVERSITY kind of regional protection available): ARIPO (BW, GH, OF CALIFORNIA [US/US]; 1111 Franklin Street, 12th GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, Floor, Oakland, California 94607-5200 (US). -

Wo 2009/120182 A2
(12) INTERNATIONALAPPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 1 October 2009 (01.10.2009) WO 2009/120182 A2 (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61K 47/34 (2006.01) CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (21) International Application Number: HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, PCT/US2008/013816 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (22) International Filing Date: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, 18 December 2008 (18.12.2008) NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, (25) Filing Language: English UG, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/015,365 20 December 2007 (20.12.2007) US GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, 12/262,986 31 October 2008 (3 1.10.2008) US ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, (71) Applicant: EASTMAN CHEMICAL COMPANY [US/ ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, US]; 200 South Wilcox Drive, Kingsport, TN 37660 MC, MT, NL, NO, PL, PT, RO, SE, SI, SK, TR), OAPI (US). -

(12) Patent Application Publication (10) Pub. No.: US 2017/0096418 A1 Patron Et Al
US 201700 96418A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0096418 A1 Patron et al. (43) Pub. Date: Apr. 6, 2017 (54) COMPOUNDS USEFUL AS MODULATORS Related U.S. Application Data OF TRPM8 (60) Provisional application No. 62/236,080, filed on Oct. (71) Applicant: Senomyx, Inc., San Diego, CA (US) 1, 2015. (72) Inventors: Andrew P. Patron, San Marcos, CA Publication Classification (US); Alain Noncovich, San Diego, CA (51) Int. Cl. (US); Jane Ung, San Diego, CA (US); C07D 409/2 (2006.01) Timothy Davis, Santee, CA (US); A6IR 9/00 (2006.01) Joseph R. Fotsing, San Diego, CA A63L/455 (2006.01) (US); Chad Priest, Leucadia, CA (US); (52) U.S. Cl. Catherine Tachdjian, San Diego, CA CPC ........ C07D 409/12 (2013.01); A61K 31/4155 (US) (2013.01); A61K 9/0014 (2013.01) (21) Appl. No.: 15/282,432 (57) ABSTRACT The present disclosure relates to compounds which are (22) Filed: Sep. 30, 2016 useful as cooling sensation compounds. US 2017/0096418 A1 Apr. 6, 2017 COMPOUNDS USEFUL AS MODULATORS -continued OF TRPM8 RELATED APPLICATIONS 0001. This application claims the benefit of U.S. Provi sional Application No. 62/236,080, filed Oct. 1, 2015, which is incorporated herein by reference in its entirety. BACKGROUND 0002 Field 0003. The present invention relates to compounds useful as cooling agents. 0004 Background Description 0005 Modulators of the Melastatin Transient Receptor or a salt or solvate thereof. Potential Channel 8 (TRPM8) are known to have a cooling effect. TRPM8 is a channel involved in the chemesthetic O008) In some embodiments the compound can have the sensation, such as cool to cold temperatures as well as the Structure sensation of known cooling agents.