US20040072900A1.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(PAC) Rev 24 Based on Applicable Aegls, Erpgs, Or Teels (Chemicals Listed by CASRN) PAC Rev 24 – August 2008
Table 3: Protective Action Criteria (PAC) Rev 24 based on applicable AEGLs, ERPGs, or TEELs (Chemicals listed By CASRN) PAC Rev 24 – August 2008 Table 3 presents a listing of chemicals and PAC data based on the Chemical Abstract Service Registry Numbers (CASRNs)1 of the chemicals. Chemicals without an identified CASRN number are issued an identification number, preceded by the letter “z,” for purposes of the PAC data set. The columns presented in Table 3 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this listing by CASRN. Chemical Name The common name of the chemical. CASRN The Chemical Abstract Service Registry Number for this chemical. TEEL-0 This is the threshold concentration below which most people will experience no appreciable risk of health effects. This PAC is always based on TEEL-0 because AEGL-0 or ERPG-0 values do not exist. PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value. PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value. PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value. Units The units for the PAC values (ppm or mg/m3). Additional information on the chemicals presented here is provided in PAC Tables 1, 2, and 4. Table 3, other PAC Tables, introductory/explanatory material (including a glossary of acronyms and abbreviations), definitions of PAC values, and alternative methods of displaying PAC information are available electronically at: http://www.hss.energy.gov/HealthSafety/WSHP/chem_safety/teel.html. -

Commission Implementing Regulation (EU)
21.3.2013 EN Official Journal of the European Union L 80/1 II (Non-legislative acts) REGULATIONS COMMISSION IMPLEMENTING REGULATION (EU) No 230/2013 of 14 March 2013 on the withdrawal from the market of certain feed additives belonging to the group of flavouring and appetising substances (Text with EEA relevance) THE EUROPEAN COMMISSION, submitted before that deadline for the only animal category for which those feed additives had been auth orised pursuant to Directive 70/524/EEC. Having regard to the Treaty on the Functioning of the European Union, (4) For transparency purposes, feed additives for which no applications for authorisation were submitted within the Having regard to Regulation (EC) No 1831/2003 of the period specified in Article 10(2) of Regulation (EC) No European Parliament and of the Council of 22 September 1831/2003 were listed in a separate part of the 2003 on additives for use in animal nutrition ( 1 ), and in Community Register of Feed Additives. particular Article 10(5) thereof, (5) Those feed additives should therefore be withdrawn from Whereas: the market as far as their use as flavouring and appetising substances is concerned, except for animal species and categories of animal species for which applications for (1) Regulation (EC) No 1831/2003 provides for the auth authorisation have been submitted. This measure does orisation of additives for use in animal nutrition and not interfere with the use of some of the abovemen for the grounds and procedures for granting such auth tioned additives according to other animal species or orisation. Article 10 of that Regulation provides for the categories of animal species or to other functional re-evaluation of additives authorised pursuant to Council groups for which they may be allowed. -

Test Items for Licensing Examination Krok 1 PHARMACY
MINISTRY OF PUBLIC HEALTH OF UKRAINE Department of human resources policy, education and science Testing Board Student ID Last name Variant ________________ Test items for licensing examination Krok 1 PHARMACY (російськомовний варіант) General Instruction Every one of these numbered questions or unfinished statements in this chapter corresponds to answers or statements endings. Choose the answer (finished statements) that fits best and fill in the circle with the corresponding Latin letter on the answer sheet. Authors of items: Abramov A.V., Aleksandrova K.V., Andronov D.Yu., Bilyk O.V., Blinder O.O., Bobyr V.V., Bobrovska O.A., Bohatyriova O.V., Bodnarchuk O.V., Boieva S.S., Bolokhovska T.O., Bondarenko Yu.I., Bratenko M.K., Buchko O.V., Cherneha H.V., Davydova N.V., Deriuhina L.I., Didenko N.O., Dmytriv A.M., Doroshkevych I.O., Dutka N.M., Dynnyk K.V., Filipova L.O., Havryliuk I.M., Herhel T.M., Hlushkova O.M., Hozhdzinsky S.M., Hrekova T.A., Hrechana O.V., Hruzevsky O.A., Hudyvok Ya.S., Hurmak I.S., Ivanets L.M., Ivanov Ye.I., Kartashova T.V., Kava T.V., Kazakova V.V., Kazmirchuk H.V., Kernychna I.Z., Khlus K.M., Khmelnykova L.I., Klebansky Ye.O., Klopotsky H.A., Klymniuk S.I., Kobylinska L.I., Koldunov V.V., Kolesnyk V.P., Kolesnikova T.O., Komlevoy O.M., Kononenko N.M., Kornijevsky Yu.Y., Kremenska L.V., Krushynska T.Yu., Kryzhanovska A.V., Kryshtal M.V., Kukurychkin Ye.R., Kuznietsova N.L., Kuzmina A.V., Lisnycha A.M., Lychko V.H., Makats Ye.F., Maly V.V., Matvijenko A.H., Menchuk K.M., Minarchenko V.M., Mikheiev A.O., Mishchenko -

56 Subpart F—Flavoring Agents and Related Substances
§ 172.510 21 CFR Ch. I (4–1–12 Edition) needed to produce its intended effect (a) They are used in the minimum but not in excess of 13 parts per million quantity required to produce their in- calculated as anhydrous sodium ferro- tended physical or technical effect and cyanide. in accordance with all the principles of [42 FR 14491, Mar. 15, 1977, as amended at 58 good manufacturing practice. FR 17098, Apr. 1, 1993] (b) In the appropriate forms (plant parts, fluid and solid extracts, con- Subpart F—Flavoring Agents and centrates, absolutes, oils, gums, bal- Related Substances sams, resins, oleoresins, waxes, and dis- tillates) they consist of one or more of § 172.510 Natural flavoring substances the following, used alone or in com- and natural substances used in con- bination with flavoring substances and junction with flavors. adjuvants generally recognized as safe Natural flavoring substances and in food, previously sanctioned for such natural adjuvants may be safely used use, or regulated in any section of this in food in accordance with the fol- part. lowing conditions. Common name Scientific name Limitations Aloe ................................................................ Aloe perryi Baker, A. barbadensis Mill., A. ferox Mill., and hybrids of this sp. with A. africana Mill. and A. spicata Baker. Althea root and flowers .................................. Althea officinalis L. Amyris (West Indian sandalwood) ................. Amyris balsamifera L. Angola weed .................................................. Roccella fuciformis -

RIFM Fragrance Ingredient Safety Assessment, Menthyl Isovalerate CAS Registry Number 16409-46-4
Food and Chemical Toxicology 110 (2017) S486eS495 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Short review RIFM fragrance ingredient safety assessment, menthyl isovalerate CAS Registry Number 16409-46-4 * A.M. Api a, , D. Belsito b, D. Botelho a, D. Browne a, M. Bruze c, G.A. Burton Jr. d, J. Buschmann e, M.L. Dagli f, M. Date a, W. Dekant g, C. Deodhar a, M. Francis a, A.D. Fryer h, K. Joshi a,S.LaCavaa, A. Lapczynski a, D.C. Liebler i, D. O'Brien a, R. Parakhia a,A.Patela, T.M. Penning j, G. Ritacco a, J. Romine a, D. Salvito a, T.W. Schultz k, I.G. Sipes l, Y. Thakkar a, E.H. Theophilus a, A.K. Tiethof a, Y. Tokura m, S. Tsang a, J. Wahler a a Research Institute for Fragrance Materials, Inc., 50 Tice Boulevard, Woodcliff Lake, NJ 07677, USA b Member RIFM Expert Panel, Columbia University Medical Center, Department of Dermatology, 161 Fort Washington Ave., New York, NY 10032, USA c Member RIFM Expert Panel, Malmo University Hospital, Department of Occupational & Environmental Dermatology, Sodra Forstadsgatan 101, Entrance 47, Malmo SE-20502, Sweden d Member RIFM Expert Panel, School of Natural Resources & Environment, University of Michigan, Dana Building G110, 440 Church St., Ann Arbor, MI 58109, USA e Member RIFM Expert Panel, Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Strasse 1, 30625 Hannover, Germany f Member RIFM Expert Panel, University of Sao Paulo, School of Veterinary Medicine and Animal Science, Department of Pathology, Av. -

2013 Pguy Acd Labs Presentation
‘Next Generation Fingerprinting’ A GC‐HR‐TOF‐MS Method to Semi‐Quantify Constituents in Aerosols and Aerosol Fractions E. Dossin, A. Monge, E. Martin, P. Pospisil, A. Knorr, M.C. Bentley, P.A. Guy Metabolomics & Analytical Chemistry Gr., Philip Morris Products SA, 2000 Neuchatel, Switzerland Outline 1. Description of the existing GC-MS fingerprinting method Pros & cons of the current fingerprinting method Why switching to a 7200 Agilent high resolution MS instrument? 2. How to tackle semi-quantification of the smoke constituents with the help of chemoinformatics tools Curation step for smoke constituents to be monitored Analysis of reference standards Retention time prediction model (QSPR approach) Selection of appropriate internal standards (clustering approach) 3. Semi-quantification from calibration curve of reference standards Linearity Assessment of silylation 4. What about other smoke constituents? 5. Conclusion Page: 2 Existing Fingerprinting Method • Aerosol sample generated from a smoking machine (ISO) – Whole smoke – Gas Vapor Phase (GVP) sbPBS – Total Particulate Matter (TPM) • Compounds list • GC columns (HP6890 GC) – DB-624: HS-SPME-GC-MS Volatile Chemicals – DB-FFAP: GC-MS Non Polar Chemicals –DB-5-MS: HT-GC-MS Polar Chemicals (TMS) • Detection (MSD5973 MS) – Electron ionization mode – Full scan (low resolution) Semi-quantification (d6-phenol) Page: 3 Description of the Former GC‐MS Fingerprinting Method (HP6890 GC ‐ MSD5973 MS) Cell culture Medium plus Cigarette DB-624 column ● Headspace(HS)-SPME-GC-MS Smoke high -

(12) United States Patent (10) Patent N0.: US 7,265,155 B2 Artman Et A1
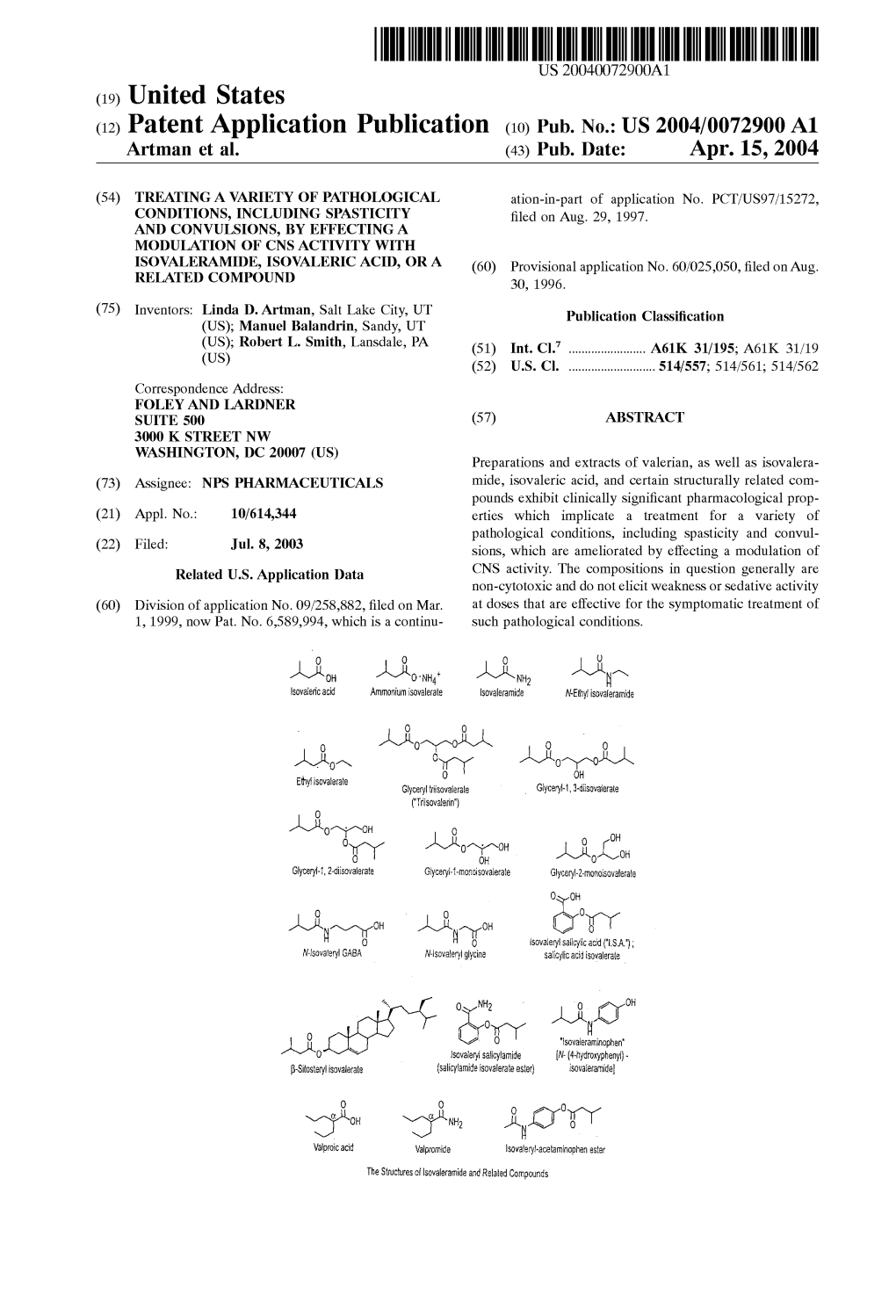
US007265155B2 (12) United States Patent (10) Patent N0.: US 7,265,155 B2 Artman et a1. (45) Date of Patent: *Sep. 4, 2007 (54) TREATING A VARIETY OF PATHOLOGICAL W0 98 08498 A 3/1998 CONDITIONS, INCLUDING SPASTICITY WO WO98/08498 3/1998 AND CONVULSIONS, BY EFFECTING A WO WO99/44623 3/1999 MODULATION OF CNS ACTIVITY WITH W0 WO 01/28516 10/2000 ISOVALERAMIDE, ISOVALERIC ACID, OR A RELATED COMPOUND OTHER PUBLICATIONS Schon and Blau, J Neurol Neurosurg Psychiatry, Sep. 1987: (75) Inventors: Linda D. Artman, Salt Lake City, UT 50(9):1148-1152.* (US); Manuel Balandrin, Sandy, UT Dorland’s Medical Dictionary 27th ed. p. 379* (US); Robert L. Smith, Lansdale, PA Pharmacotherapy, A Pathophysiologic Approach, Dipiro et al.2nd (Us) ed. 1991, pp. 1232, 1238).* Drug facts and comparisons 1999 ed. pp. 1595-1597(“Drug (73) Assignee: NBS Pharmaceuticals, Inc., Salt Lake Facts”).* City, UT (US) Pharmacotherapy, A Pathophysiologic Approach, Dipiro et al. 2nd ed. 1991, pp. 1232 & 1238* Notice: Subject to any disclaimer, the term of this Drug Facts and Comparisons. 1999 ed. pp. 1595-1597 (“Drug patent is extended or adjusted under 35 Facts”).* Julius A. Vida “Advances in Anticonvulsant Drug Development”; USC 154(b) by 0 days. Anticonvulsants (1997) pp. 1-9; Academic Press. Julius A. Vida “Noncyclic Anticonvulsants”; Anticonvulsants This patent is subject to a terminal dis (1977) pp. 577-619; Academic Press. claimer. Salim Hadad, et al. “Pharmacokinetic Analysis and Antiepileptic Activity of N-Valproyl Deriatives of GABA and Glycine”; Phar (21) Appl. N0.: 10/614,344 maceutical Research (1995), pp. 905-907; Plenum Publishing Cor poration. -

Cigarette Additives, Carcinogens and Chemicals Nicotine
Cigarette Additives, Carcinogens and Chemicals Nicotine A Destructive Natural Pesticide Which ... Is extremely addictive when smoked Is extremely addictive when chewed Causes addiction as permanent as Is harder to quit than heroin or cocaine alcoholism Is not medicine and its use not therapy Is ineffective as a stand-alone quitting aid Prevents pre-cancerous cells from dying Accelerates cancer tumor growth rates Contributes to artery hardening Has a metabolite which may cause cancer May kill brain cells and impair memory Is linked to lung cancer Likely causes brain damage and Is also a fetus destroying teratogen depression Kills half of adult smokers 13-14 years Is beat by never taking another puff or early chew! 81 Cancer Causing Chemicals Have So Far Been Identified in Cigarettes Acetaldehyde Acetamide Acrylamide Acrylonitrile 2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline (MeIQ) 3-Amino-1,4-dimethyl-5H-pyrido [4,3-b]indole (Trp-P-1) 2-Amino-l-methyl-6-phenyl-1H-imidazo [4,5-b]pyridine (PhlP) 2-Amino-6-methyldipyrido[1,2-a:3',2'-d]imidazole (Glu-P-1) 3-Amino-l-methyl-5H-pyrido {4,3-b]indole (Trp-P-2 2-Amino-3-methyl-9H-pyrido[2,3-b]indole (MeAaC) 2-Amino-9H-pyrido[2,3-b]indole (AaC) 4-Aminobiphenyl 2-Aminodipyrido[1,2-a:3',2'-d]imidazole (Glu-P-2) 0-Anisidine Arsenic Benz[a]anthracene Benzene Benzo[a]pyrene Benzo[b]fluoranthene Benzo[j]fluoranthene Benzo[k]fluoranthene Benzo[b]furan Beryllium 1,3-Butadiene Cadmium Catechol (1,2-benzenediol) p-Chloroaniline Chloroform Cobalt p,p'-DDT Dibenz[a,h]acridine Dibenz[a,j]acridine Dibenz(a,h)anthracene -

Cover Next Page > Cover Next Page >
cover next page > Cover title: The Psychopharmacology of Herbal Medicine : Plant Drugs That Alter Mind, Brain, and Behavior author: Spinella, Marcello. publisher: MIT Press isbn10 | asin: 0262692651 print isbn13: 9780262692656 ebook isbn13: 9780585386645 language: English subject Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. publication date: 2001 lcc: RC483.S65 2001eb ddc: 615/.788 subject: Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. cover next page > < previous page page_i next page > Page i The Psychopharmacology of Herbal Medicine < previous page page_i next page > cover next page > Cover title: The Psychopharmacology of Herbal Medicine : Plant Drugs That Alter Mind, Brain, and Behavior author: Spinella, Marcello. publisher: MIT Press isbn10 | asin: 0262692651 print isbn13: 9780262692656 ebook isbn13: 9780585386645 language: English subject Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. publication date: 2001 lcc: RC483.S65 2001eb ddc: 615/.788 subject: Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. cover next page > < previous page page_ii next page > Page ii This page intentionally left blank. < previous page page_ii next page > < previous page page_iii next page > Page iii The Psychopharmacology of Herbal Medicine Plant Drugs That Alter Mind, Brain, and Behavior Marcello Spinella < previous page page_iii next page > < previous page page_iv next page > Page iv © 2001 Massachusetts Institute of Technology All rights reserved. No part of this book may be reproduced in any form by any electronic or mechanical means (including photocopying, recording, or information storage and retrieval) without permission in writing from the publisher. This book was set in Adobe Sabon in QuarkXPress by Asco Typesetters, Hong Kong and was printed and bound in the United States of America. -

395 B 536 Winstal. O. Saito EEEEEE
USOO8426439B2 (12) UnitedO States Patent (10) Patent No.: US 8.426,439 B2 Ciccocioppo (45) Date of Patent: *Apr. 23, 2013 (54) COMPOSITIONS AND METHODS FOR 7,510,728 B2 3/2009 Koike ........................... 424/464 PROPHYLAXIS AND TREATMENT OF 7,517,900 B2 4/2009 Pendri et al. ... 514,404 7,524,975 B2 4/2009 Mae et al. ... 549,405 ADDCTIONS 2002fOOO6942 A1 1/2002 Davis ........... ... 514,369 2002fOO77320 A1 6/2002 Lohray et al. 514/226.2 (75) Inventor: Roberto Ciccocioppo, Camerino (IT) 2003/0069246 A1 4/2003 Darrow et al. ................ 514,245 2003/010.0587 A1 5/2003 Moinet et al. ................. 514,369 (73) Assignee: Omeros Corporation, Seattle, WA (US) 2003/0220373 Al 1 1/2003 Jaye et al. ... 514,342 2004/0028735 A1 2/2004 Kositprapa ... 424/468 2004/OO77525 A1 4/2004 Chapman et al. ... 514/2 - r 2004/0096499 A1 5/2004 Vaya et al. ... 424/468 (*) Notice: Subject to any disclaimer, the term of this 2004/0127443 A1 7/2004 Pershadsingh 514,44 patent is extended or adjusted under 35 2004/0204472 A1 10/2004 Briggs et al. 514,406 U.S.C. 154(b) by 859 days. 2005, 00041 79 A1 1/2005 Pedersen ...... ... 514,342 2005, OO14786 A1 1/2005 Sun et al. .. ... 514/313 This patent is Subject to a terminal dis- 2005, OO 14833 A1 1/2005 Clark et al. .. ... 514,561 claimer. 2005, 0096331 A1 5/2005 Das et al. 514,259.3 2005, 01711.1.0 A1 8, 2005 Yu et al. ....... ... 514,248 2006, OOO9518 A1 1/2006 Campbell et al. -

Thermochemical Properties and Regularities of Amides, Anilides
DOI: 10.17344/acsi.2017.3683 Acta Chim. Slov. 2018, 65, 127–130 127 Scientific paper Thermochemical Properties and Regularities of Amides, Anilides, and Amidic Acids Alma Kairlapovna Ryskaliyeva,* Murat Ergalievich Baltabayev and Aigul Moldakhmetovna Zhubatova Kazakh National Agrarian University, 8 Abay Av., 050000 Almaty, Kazakhstan * Corresponding author: E-mail: [email protected] Received: 08-07-2017 Abstract The thermodynamic properties of carbamide and its alkyl substituted were studied insufficiently. In this article, the en- thalpies of combustion of ΔсН of some amides, anilides and amidic acids have been determined experimentally; their standard enthalpies of formation have been calculated. Linear dependences between the average atomic enthalpy of + combustion of ΔcH amides and their basicity constants in acidic aqueous solutions of pKBH have been established; a correlation that relates the enthalpies of combustion of amides and the corresponding amidic acids has been found. Keywords: Enthalpy of combustion, thermochemistry, amides, correlation analysis 3 1. Introduction self-packing calorimetric bomb (Vint. = 325 cm ), equipped with two valves (for input and output of gases), was used to Amides, anilides and their derivatives play an im- determine the enthalpies of combustion of the studied portant role in various biochemical processes and are compounds. The measurement error of the calorimeter therefore widely used as analytical and organic reagents in was ± 0.1%, which was absolutely insufficient to make pre- plant growing, -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Rhubarb, garden root ..................................... Rheum rhaponticum L ................................................ Do. Rhubarb root .................................................. Rheum officinale Baill., R. palmatum L., or other spp. (excepting R. rhaponticum L.) or hybrids of Rheum grown in China. Roselle ........................................................... Hibiscus sabdariffa L .................................................. Do. Rosin (colophony) .......................................... Pinus palustris Mill., and other Pinus spp .................. Do. St. Johnswort leaves, flowers, and caulis ...... Hypericum perforatum L ............................................. Hypericin-free alcohol dis- tillate form only; in alco- holic beverages only Sandalwood, white (yellow, or East Indian) ... Santalum album L. Sandarac ........................................................ Tetraclinis articulata (Vahl.), Mast .............................. In alcoholic beverages only Sarsaparilla ..................................................... Smilax aristolochiaefolia Mill., (Mexican sarsaparilla), S. regelii Killip et Morton (Honduras sarsaparilla), S. febrifuga Kunth (Ecuadorean sarsaparilla), or undetermined Smilax spp. (Ecuadorean or Central American sarsaparilla). Sassafras leaves ............................................ Sassafras albidum (Nutt.) Nees ................................. Safrole free Senna, Alexandria .........................................