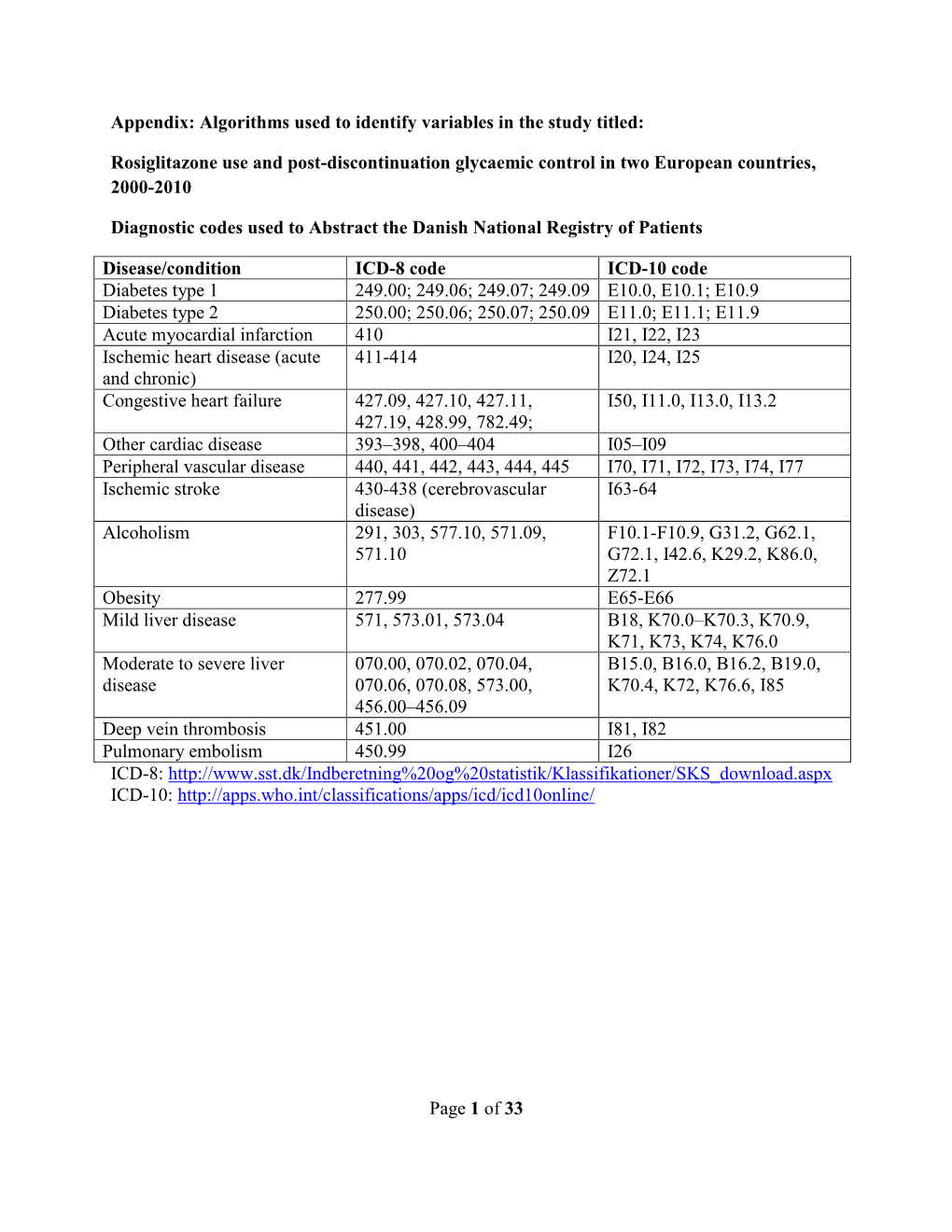
Of 33 Appendix
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Topical Beta-Adrenoceptor Blocking Agents on Pulsatile Ocular Blood Flow
THE EFFECT OF TOPICAL BETA-ADRENOCEPTOR BLOCKING AGENTS ON PULSATILE OCULAR BLOOD FLOW C. D. MORSMAN, M. E. BOSEM, M. LUSKY and R. N. WEINREB San Diego, California SUMMARY factors (e.g. hypertension, diabetes, peripheral Thirty-three ocular hypertensive patients (21 with vascular disease and vasospasm) to the vascular primary open angle glaucoma and 12 glaucoma system suggest that blood flow in the optic nerve suspects) were randomly assigned to receive either head and retina may be altered in glaucoma.4 Of the timolol, levobunolol or betaxolol in one eye. Pulsatile various vascular beds within the posterior segment, ocular blood flow (POBF) was measured before the choroidal circulation is of particular interest since treatment (baseline) and 2 hours after drop adminis it provides the major contribution to the blood flow tration. After 1 week of regular twice-daily dosage, of the optic nerve at the level of the lamina cribrosa.5 POBF was measured again both immediately before Beta-2 adrenergic receptors have been demon and 2 hours after drop instillation. All measurements strated in human optic nerve,6 as well as in choroidal were made by an investigator masked to treatment. and retinal blood vessels.7 Blockade of these POBF increased by 11% (p = 0.09) at week 0 after receptors can cause vasoconstrictionS which could levobunolol administration, and by 22% (p = 0.20) at adversely affect visual function if adequate concen week 1 before drop administration compared with trations of the drug diffused into the posterior baseline. It dropped by 23% and 25% (p = 0.04 and segment of the eye or were absorbed systemically. -

Documentationand Coding Tips: Peripheral Vascular Disease
Documentation and Coding: Peripheral Vascular Disease (PVD)/ Peripheral Arterial Disease (PAD) Created May 2020 At Healthfirst, we are committed to helping providers accurately document and code their patients’ health records. Proper ICD-10 coding can provide a comprehensive view of a patient’s overall health. This tip sheet offers guidance on how to submit a diagnosis code with greater specificity for Peripheral Vascular Disease (PVD)/Peripheral Arterial Disease (PAD). The risk factors for peripheral vascular disease are similar to those for coronary artery disease. The terms arteriosclerosis and atherosclerosis may be used interchangeably for coding and documentation purposes. When completing documentation and coding, you should keep in mind the following: Type of graft Laterality (left, right, or bilateral) and side(s) affected by the complicating condition Type of bypass Any educational information provided to the patient Location of vein or artery graft affected Treatment plan, orders, testing, prescriptions, and referrals Complications like claudication, ulceration, or chest pain Updated status of condition (stable, improved, and/or worsening) Graft, Bypass, Location, Complications, and Laterality Use the chart below to ensure that you are coding properly. Note that this is not an inclusive list of codes and that you are required to have six digits for your diagnosis code. The location and laterality will help determine the fifth and sixth digits for the codes. Type of Graft Location/Laterality Description of Graft ICD-10-CM Without -

In Silico Methods for Drug Repositioning and Drug-Drug Interaction Prediction
In silico Methods for Drug Repositioning and Drug-Drug Interaction Prediction Pathima Nusrath Hameed ORCID: 0000-0002-8118-9823 Submitted in total fulfilment of the requirements for the degree of Doctor of Philosophy Department of Mechanical Engineering THE UNIVERSITY OF MELBOURNE May 2018 Copyright © 2018 Pathima Nusrath Hameed All rights reserved. No part of the publication may be reproduced in any form by print, photoprint, microfilm or any other means without written permission from the author. Abstract Drug repositioning and drug-drug interaction (DDI) prediction are two fundamental ap- plications having a large impact on drug development and clinical care. Drug reposi- tioning aims to identify new uses for existing drugs. Moreover, understanding harmful DDIs is essential to enhance the effects of clinical care. Exploring both therapeutic uses and adverse effects of drugs or a pair of drugs have significant benefits in pharmacology. The use of computational methods to support drug repositioning and DDI prediction en- able improvements in the speed of drug development compared to in vivo and in vitro methods. This thesis investigates the consequences of employing a representative training sam- ple in achieving better performance for DDI classification. The Positive-Unlabeled Learn- ing method introduced in this thesis aims to employ representative positives as well as reliable negatives to train the binary classifier for inferring potential DDIs. Moreover, it explores the importance of a finer-grained similarity metric to represent the pairwise drug similarities. Drug repositioning can be approached by new indication detection. In this study, Anatomical Therapeutic Chemical (ATC) classification is used as the primary source to determine the indications/therapeutic uses of drugs for drug repositioning. -

Apo-Cilazapril/Hydrochlorothiazide Film Coated Tablet
New Zealand Data Sheet APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE 1. PRODUCT NAME APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE – cilazapril 5mg and hydrochlorothiazide 12.5mg film coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Cilazapril monohydrate 5.22mg (equivalent to Cilazapril 5mg) and Hydrochlorothiazide 12.5mg Excipient(s) with known effect HYDROCHLOROTHIAZIDE contains sulphur. APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is lactose free and gluten free. APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE contains Red Ferric Oxide (orange shade # 34690). For the full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE are pink, oval biconvex film-coated tablets. Each tablet is engraved “APO” on one side and “5” bisect “12.5” on the other side. Each tablet typically weighs 92mg. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is indicated for the treatment of patients with hypertension who are not adequately controlled on monotherapy. 4.2 Dose and method of administration Standard Dosage The dosage of APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is one tablet administered once daily. As food intake has no clinically significant influence on absorption, APO- CILAZAPRIL/HYDROCHLOROTHIAZIDE can be administered before or after meals. The dose should always be taken at about the same time of day. Special Populations Renal insufficiency When concomitant diuretic therapy is required in patients with severe renal impairment, a loop diuretic rather than a thiazide diuretic is preferred for use with cilazapril/hydrochlorothiazide; therefore, for patients with severe renal dysfunction (creatinine Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 1 of 22 APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE clearance <10ml/min), APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is not recommended. -

Pediatric Pharmacotherapy
Pediatric Pharmacotherapy A Monthly Review for Health Care Professionals of the Children's Medical Center Volume 1, Number 10, October 1995 DIURETICS IN CHILDREN • Overview • Loop Diuretics • Thiazide Diuretics • Metolazone • Potassium Sparing Diuretics • Diuretic Dosages • Efficacy of Diuretics in Chronic Pulmonary Disease • Summary • References Pharmacology Literature Reviews • Ibuprofen Overdosage • Predicting Creatinine Clearance Formulary Update Diuretics are used for a wide variety of conditions in infancy and childhood, including the management of pulmonary diseases such as respiratory distress syndrome (RDS) and bronchopulmonary dysplasia (BPD)(1 -5). Both RDS and BPD are often associated with underlying pulmonary edema and clinical improvement has been documented with diuretic use.6 Diuretics also play a major role in the management of congestive heart failure (CHF), which is frequently the result of congenital heart disease (7). Other indications, include hypertension due to the presence of cardiac or renal dysfunction. Hypertension in children is often resistant to therapy, requiring the use of multidrug regimens for optimal blood pressure control (8). Control of fluid and electrolyte status in the pediatric population remains a therapeutic challenge due to the profound effects of age and development on renal function. Although diuretics have been used extensively in infants and children, few controlled studies have been conducted to define the pharmacokinetics and pharmacodynamics of diuretics in this population. Nonetheless, diuretic therapy has become an important part of the management of critically ill infants and children. This issue will review the mechanisms of action, monitoring parameters, and indications for use of diuretics in the pediatric population (1-5). Loop Diuretics Loop diuretics are the most potent of the available diuretics (4). -

The Role of Non-Selective Β-Blockers in Compensated Cirrhotic Patients Without Major Complications
medicina Article The Role of Non-Selective β-Blockers in Compensated Cirrhotic Patients without Major Complications Wen-Shuo Yeh 1, Shih-Cheng Yang 1, Chih-Ming Liang 1 , Yu-Chi Li 1, Wei-Chen Tai 1, Chen-Hsiang Lee 2, Yao-Hsu Yang 3,4,5, Chien-Ning Hsu 6,7, Tzu-Hsien Tsai 8, Seng-Kee Chuah 1 and Cheng-Kun Wu 1,* 1 Division of Hepato-Gastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 83330, Taiwan; [email protected] (W.-S.Y.); [email protected] (S.-C.Y.); [email protected] (C.-M.L.); [email protected] (Y.-C.L.); [email protected] (W.-C.T.); [email protected] (S.-K.C.) 2 Division of Infectious Diseases, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 83330, Taiwan; [email protected] 3 Department of Traditional Chinese Medicine, Chiayi Chang Gung Memorial Hospital, Chiayi 61363, Taiwan; [email protected] 4 Health Information and Epidemiology Laboratory of Chang Gung Memorial Hospital, Chiayi 61363, Taiwan 5 School of Traditional Chinese Medicine, College of Medicine, Chang Gung University, Taoyuan 33302, Taiwan 6 Department of Pharmacy, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung 83330, Taiwan; [email protected] 7 School of Pharmacy, Kaohsiung Medical University, Kaohsiung 80700, Taiwan 8 Division of Cardiology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 83330, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-7-731-7123 (ext. -

Interactions Medicamenteuses Index Des Classes Pharmaco
INTERACTIONS MEDICAMENTEUSES INDEX DES CLASSES PHARMACO-THERAPEUTIQUES Mise à jour avril 2006 acides biliaires (acide chenodesoxycholique, acide ursodesoxycholique) acidifiants urinaires adrénaline (voie bucco-dentaire ou sous-cutanée) (adrenaline alcalinisants urinaires (acetazolamide, sodium (bicarbonate de), trometamol) alcaloïdes de l'ergot de seigle dopaminergiques (bromocriptine, cabergoline, lisuride, pergolide) alcaloïdes de l'ergot de seigle vasoconstricteurs (dihydroergotamine, ergotamine, methylergometrine) alginates (acide alginique, sodium et de trolamine (alginate de)) alphabloquants à visée urologique (alfuzosine, doxazosine, prazosine, tamsulosine, terazosine) amidons et gélatines (gelatine, hydroxyethylamidon, polygeline) aminosides (amikacine, dibekacine, gentamicine, isepamicine, kanamycine, netilmicine, streptomycine, tobramycine) amprénavir (et, par extrapolation, fosamprénavir) (amprenavir, fosamprenavir) analgésiques morphiniques agonistes (alfentanil, codeine, dextromoramide, dextropropoxyphene, dihydrocodeine, fentanyl, hydromorphone, morphine, oxycodone, pethidine, phenoperidine, remifentanil, sufentanil, tramadol) analgésiques morphiniques de palier II (codeine, dextropropoxyphene, dihydrocodeine, tramadol) analgésiques morphiniques de palier III (alfentanil, dextromoramide, fentanyl, hydromorphone, morphine, oxycodone, pethidine, phenoperidine, remifentanil, sufentanil) analogues de la somatostatine (lanreotide, octreotide) androgènes (danazol, norethandrolone, testosterone) anesthésiques volatils halogénés -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Summary of Product Characteristics
Proposed var 24 psusa cilazapril SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [Fosinopril sodium 10 mg, tablets] [Fosinopril sodium 20 mg, tablets] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 or 20 mg fosinopril sodium. Excipient with known effect: Each tablet fosinopril sodium 10 mg contains 87 mg of lactose, anhydrous. Each tablet fosinopril sodium 20 mg contains 174 mg of lactose, anhydrous. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. The 10 mg tablets are white and shaped like a capsule with indents. On one side they are engraved with the letters “APO” and on the other side with “FOS-10”. The 20 mg tablets are white and their shape is oval. On one side they are engraved with the letters “APO” and on the other side with “FOS-20”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Treatment of hypertension. - Treatment of symptomatic heart failure. 4.2 Posology and method of administration Posology Fosinopril sodium should be administered orally in a single daily dose. As with all other medicinal products taken once daily, it should be taken at approximately the same time each day. The absorption of fosinopril sodium is not affected by food. The dose should be individualised according to patient profile and blood pressure response (see section 4.4). Hypertension: Fosinopril sodium may be used as a monotherapy or in combination with other classes of antihypertensive medicinal products (see section 4.3, 4.4, 4.5 and 5.1). Hypertensive patients not being treated with diuretics: Starting dose The initial recommended dose is 10 mg once a day. -

Use of Emulsions for Intra- and Periocular Injection
(19) & (11) EP 1 611 879 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/107 (2006.01) 12.08.2009 Bulletin 2009/33 (21) Application number: 04291684.1 (22) Date of filing: 02.07.2004 (54) Use of emulsions for intra- and periocular injection Verwendung von Emulsionen zur intra- und periocularen Injection Utilisation des émulsions pour injection intra- et périoculaire. (84) Designated Contracting States: (74) Representative: de Mareüil-Villette, Caroline et al AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Cabinet Plasseraud HU IE IT LI LU MC NL PL PT RO SE SI SK TR 52 rue de la Victoire 75440 Paris Cedex 09 (FR) (43) Date of publication of application: 04.01.2006 Bulletin 2006/01 (56) References cited: EP-A- 0 521 799 EP-A- 1 020 194 (73) Proprietors: WO-A-02/09667 WO-A-93/18852 • Novagali Pharma S.A. WO-A-94/05298 WO-A-03/053405 91000 Evry (FR) US-A- 5 632 984 • CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS) • KLANG S H ET AL: "PHYSICOCHEMICAL 75016 Paris (FR) CHARACTERIAZATION AND ACUTE TOXICITY • INSTITUT NATIONAL DE LA SANTE ET DE LA EVALUATION OF A POSITIVELY-CHARGED RECHERCHE MEDICALE (INSERM) SUBMICRON EMULSION VEHICLE" JOURNAL 75013 Paris (FR) OF PHARMACY AND PHARMACOLOGY, • YISSUM RESEARCH DEVELOPMENT COMPANY LONDON, GB, vol. 46, no. 12, December 1994 OF THE HEBREW UNIVERSITY OF JERUSALEM (1994-12), pages 986-993, XP008005426 ISSN: 91390 Jerusalem (IL) 0022-3573 • KLANG S ET AL: "INFLUENCE OF EMULSION (72) Inventors: DROPLET SURFACE CHARGE ON • Rabinovich-Guilatt, Laura INDOMETHACIN OCULAR TISSUE 75015 Paris (FR) DISTRIBUTION" PHARMACEUTICAL • De Kozak, Yvonne DEVELOPMENT AND TECHNOLOGY, NEW 75013 Paris (FR) YORK, NY, US, vol. -

Ace Inhibitors (Angiotensin-Converting Enzyme)
Medication Instructions Ace Inhibitors (Angiotensin-Converting Enzyme) Generic Brand Benazepril Lotensin Captopril Capoten Enalapril Vasotec Fosinopril Monopril Lisinopril Prinivil, Zestril Do not Moexipril Univasc Quinapril Accupril stop taking Ramipril Altace this medicine Trandolapril Mavik About this Medicine unless told ACE inhibitors are used to treat both high blood pressure (hypertension) and heart failure (HF). They block an enzyme that causes blood vessels to constrict. This to do so allows the blood vessels to relax and dilate. Untreated, high blood pressure can damage to your heart, kidneys and may lead to stroke or heart failure. In HF, using by your an ACE inhibitor can: • Protect your heart from further injury doctor. • Improve your health • Reduce your symptoms • Can prevent heart failure. Generic forms of ACE Inhibitors (benazepril, captopril, enalapril, fosinopril, and lisinopril) may be purchased at a lower price. There are no “generics” for Accupril, Altace Mavik, and of Univasc. Thus their prices are higher. Ask your doctor if one of the generic ACE Inhibitors would work for you. How to Take Use this drug as directed by your doctor. It is best to take these drugs, especially captopril, on an empty stomach one hour before or two hours after meals (unless otherwise instructed by your doctor). Side Effects Along with needed effects, a drug may cause some unwanted effects. Many people will not have any side effects. Most of these side effects are mild and short-lived. Check with your doctor if any of the following side effects occur: • Fever and chills • Hoarseness • Swelling of face, mouth, hands or feet or any trouble in swallowing or breathing • Dizziness or lightheadedness (often a problem with the first dose) Report these side effects if they persist: • Cough – dry or continuing • Loss of taste, diarrhea, nausea, headache or unusual fatigue • Fast or irregular heartbeat, dizziness, lightheadedness • Skin rash Special Guidelines • Sodium in the diet may cause you to retain fluid and increase your blood pressure. -

33-Adrenoceptor-Mediated Relaxation Induced by Isoprenaline And
J. Smooth Muscle Res. 33 : 99-106. 99 The )32 and [33-Adrenoceptor-Mediated Relaxation Induced by Isoprenaline and Salbutamol in Guinea Pig Taenia Caecum Katsuo KOIKE, Tsukasa IcHiNo, Takahiro HORINOUCHI and Issei TAKAYANAGI Departmentof Chemical Pharmacology, Toho University School of PharmaceuticalSciences, 2-2-1, Miyama,Funabashi, Chiba 274, Japan Abstract To understand the receptor subtypes responsible for /3adrenoceptormediated relaxa tion of guinea pig taenia caecum, we investigated the effects of isoprenaline and salbutamol . Isoprenaline and salbutamol caused dose-dependent relaxation of the guinea pig taenia caecum. Propranolol, bupranolol and butoxamine produced shifts of the concentration response curves for isoprenaline and salbutamol. Schild regression analyses carried out for propranolol against isoprenaline and salbutamol gave pA2 values of 8.43 and 8.88, respective ly. Schild regression analyses carried out for butoxamine against isoprenaline and sal butamol gave pA2 values of 6.46 and 6.68, respectively. Schild regression analyses carried out for bupranolol against isoprenaline and salbutamol gave pA2 values of 8.60 and 8.69, respectively. However, in the presence of 3 x 10' M atenolol, 10-4 M butoxamine and 10-6 M phentolamine to block the fir , /32 and a -adrenoceptor effects, respectively, Schild regression analyses carried out for bupranolol against isoprenaline and salbutamol gave pA2 values of 5.77 and 5.97, respectively. These results suggest that the relaxant responses to isoprenaline and salbutamol in the guinea pig taenia caecum are mediated by both the /.32 and the A-adrenoceptors. Key words : f2-adrenoceptor, A-adrenoceptor, isoprenaline, salbutamol , guinea pig taenia caecum Introduction The /3adrenoceptors were subclassified as and /32subtypes based on the agonist potency and tissue localization.