Novel Method of Administering Β-Blockers and Novel Dosage Forms Containing Same Anwar A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 9,498,481 B2 Rao Et Al
USOO9498481 B2 (12) United States Patent (10) Patent No.: US 9,498,481 B2 Rao et al. (45) Date of Patent: *Nov. 22, 2016 (54) CYCLOPROPYL MODULATORS OF P2Y12 WO WO95/26325 10, 1995 RECEPTOR WO WO99/O5142 2, 1999 WO WOOO/34283 6, 2000 WO WO O1/92262 12/2001 (71) Applicant: Apharaceuticals. Inc., La WO WO O1/922.63 12/2001 olla, CA (US) WO WO 2011/O17108 2, 2011 (72) Inventors: Tadimeti Rao, San Diego, CA (US); Chengzhi Zhang, San Diego, CA (US) OTHER PUBLICATIONS Drugs of the Future 32(10), 845-853 (2007).* (73) Assignee: Auspex Pharmaceuticals, Inc., LaJolla, Tantry et al. in Expert Opin. Invest. Drugs (2007) 16(2):225-229.* CA (US) Wallentin et al. in the New England Journal of Medicine, 361 (11), 1045-1057 (2009).* (*) Notice: Subject to any disclaimer, the term of this Husted et al. in The European Heart Journal 27, 1038-1047 (2006).* patent is extended or adjusted under 35 Auspex in www.businesswire.com/news/home/20081023005201/ U.S.C. 154(b) by Od en/Auspex-Pharmaceuticals-Announces-Positive-Results-Clinical M YW- (b) by ayS. Study (published: Oct. 23, 2008).* This patent is Subject to a terminal dis- Concert In www.concertpharma. com/news/ claimer ConcertPresentsPreclinicalResultsNAMS.htm (published: Sep. 25. 2008).* Concert2 in Expert Rev. Anti Infect. Ther. 6(6), 782 (2008).* (21) Appl. No.: 14/977,056 Springthorpe et al. in Bioorganic & Medicinal Chemistry Letters 17. 6013-6018 (2007).* (22) Filed: Dec. 21, 2015 Leis et al. in Current Organic Chemistry 2, 131-144 (1998).* Angiolillo et al., Pharmacology of emerging novel platelet inhibi (65) Prior Publication Data tors, American Heart Journal, 2008, 156(2) Supp. -

United States Patent (10) Patent No.: US 8,969,514 B2 Shailubhai (45) Date of Patent: Mar
USOO896.9514B2 (12) United States Patent (10) Patent No.: US 8,969,514 B2 Shailubhai (45) Date of Patent: Mar. 3, 2015 (54) AGONISTS OF GUANYLATECYCLASE 5,879.656 A 3, 1999 Waldman USEFUL FOR THE TREATMENT OF 36; A 6. 3: Watts tal HYPERCHOLESTEROLEMIA, 6,060,037- W - A 5, 2000 Waldmlegand et al. ATHEROSCLEROSIS, CORONARY HEART 6,235,782 B1 5/2001 NEW et al. DISEASE, GALLSTONE, OBESITY AND 7,041,786 B2 * 5/2006 Shailubhai et al. ........... 530.317 OTHER CARDOVASCULAR DISEASES 2002fOO78683 A1 6/2002 Katayama et al. 2002/O12817.6 A1 9/2002 Forssmann et al. (75) Inventor: Kunwar Shailubhai, Audubon, PA (US) 2003,2002/0143015 OO73628 A1 10/20024, 2003 ShaubhaiFryburg et al. 2005, OO16244 A1 1/2005 H 11 (73) Assignee: Synergy Pharmaceuticals, Inc., New 2005, OO32684 A1 2/2005 Syer York, NY (US) 2005/0267.197 A1 12/2005 Berlin 2006, OO86653 A1 4, 2006 St. Germain (*) Notice: Subject to any disclaimer, the term of this 299;s: A. 299; NS et al. patent is extended or adjusted under 35 2008/0137318 A1 6/2008 Rangarajetal.O U.S.C. 154(b) by 742 days. 2008. O151257 A1 6/2008 Yasuda et al. 2012/O196797 A1 8, 2012 Currie et al. (21) Appl. No.: 12/630,654 FOREIGN PATENT DOCUMENTS (22) Filed: Dec. 3, 2009 DE 19744O27 4f1999 (65) Prior Publication Data WO WO-8805306 T 1988 WO WO99,26567 A1 6, 1999 US 2010/O152118A1 Jun. 17, 2010 WO WO-0 125266 A1 4, 2001 WO WO-02062369 A2 8, 2002 Related U.S. -

1-(4-Amino-Cyclohexyl)
(19) & (11) EP 1 598 339 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 211/04 (2006.01) C07D 211/06 (2006.01) 24.06.2009 Bulletin 2009/26 C07D 235/24 (2006.01) C07D 413/04 (2006.01) C07D 235/26 (2006.01) C07D 401/04 (2006.01) (2006.01) (2006.01) (21) Application number: 05014116.7 C07D 401/06 C07D 403/04 C07D 403/06 (2006.01) A61K 31/44 (2006.01) A61K 31/48 (2006.01) A61K 31/415 (2006.01) (22) Date of filing: 18.04.2002 A61K 31/445 (2006.01) A61P 25/04 (2006.01) (54) 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE DERIVATIVES AND RELATED COMPOUNDS AS NOCICEPTIN ANALOGS AND ORL1 LIGANDS FOR THE TREATMENT OF PAIN 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ON DERIVATE UND VERWANDTE VERBINDUNGEN ALS NOCICEPTIN ANALOGE UND ORL1 LIGANDEN ZUR BEHANDLUNG VON SCHMERZ DERIVÉS DE LA 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE ET COMPOSÉS SIMILAIRES POUR L’UTILISATION COMME ANALOGUES DU NOCICEPTIN ET LIGANDES DU ORL1 POUR LE TRAITEMENT DE LA DOULEUR (84) Designated Contracting States: • Victory, Sam AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Oak Ridge, NC 27310 (US) MC NL PT SE TR • Whitehead, John Designated Extension States: Newtown, PA 18940 (US) AL LT LV MK RO SI (74) Representative: Maiwald, Walter (30) Priority: 18.04.2001 US 284666 P Maiwald Patentanwalts GmbH 18.04.2001 US 284667 P Elisenhof 18.04.2001 US 284668 P Elisenstrasse 3 18.04.2001 US 284669 P 80335 München (DE) (43) Date of publication of application: (56) References cited: 23.11.2005 Bulletin 2005/47 EP-A- 0 636 614 EP-A- 0 990 653 EP-A- 1 142 587 WO-A-00/06545 (62) Document number(s) of the earlier application(s) in WO-A-00/08013 WO-A-01/05770 accordance with Art. -

Interactions Medicamenteuses Index Des Classes Pharmaco
INTERACTIONS MEDICAMENTEUSES INDEX DES CLASSES PHARMACO-THERAPEUTIQUES Mise à jour avril 2006 acides biliaires (acide chenodesoxycholique, acide ursodesoxycholique) acidifiants urinaires adrénaline (voie bucco-dentaire ou sous-cutanée) (adrenaline alcalinisants urinaires (acetazolamide, sodium (bicarbonate de), trometamol) alcaloïdes de l'ergot de seigle dopaminergiques (bromocriptine, cabergoline, lisuride, pergolide) alcaloïdes de l'ergot de seigle vasoconstricteurs (dihydroergotamine, ergotamine, methylergometrine) alginates (acide alginique, sodium et de trolamine (alginate de)) alphabloquants à visée urologique (alfuzosine, doxazosine, prazosine, tamsulosine, terazosine) amidons et gélatines (gelatine, hydroxyethylamidon, polygeline) aminosides (amikacine, dibekacine, gentamicine, isepamicine, kanamycine, netilmicine, streptomycine, tobramycine) amprénavir (et, par extrapolation, fosamprénavir) (amprenavir, fosamprenavir) analgésiques morphiniques agonistes (alfentanil, codeine, dextromoramide, dextropropoxyphene, dihydrocodeine, fentanyl, hydromorphone, morphine, oxycodone, pethidine, phenoperidine, remifentanil, sufentanil, tramadol) analgésiques morphiniques de palier II (codeine, dextropropoxyphene, dihydrocodeine, tramadol) analgésiques morphiniques de palier III (alfentanil, dextromoramide, fentanyl, hydromorphone, morphine, oxycodone, pethidine, phenoperidine, remifentanil, sufentanil) analogues de la somatostatine (lanreotide, octreotide) androgènes (danazol, norethandrolone, testosterone) anesthésiques volatils halogénés -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

(12) United States Patent (10) Patent No.: US 7.803,838 B2 Davis Et Al
USOO7803838B2 (12) United States Patent (10) Patent No.: US 7.803,838 B2 Davis et al. (45) Date of Patent: Sep. 28, 2010 (54) COMPOSITIONS COMPRISING NEBIVOLOL 2002fO169134 A1 11/2002 Davis 2002/0177586 A1 11/2002 Egan et al. (75) Inventors: Eric Davis, Morgantown, WV (US); 2002/0183305 A1 12/2002 Davis et al. John O'Donnell, Morgantown, WV 2002/0183317 A1 12/2002 Wagle et al. (US); Peter Bottini, Morgantown, WV 2002/0183365 A1 12/2002 Wagle et al. (US) 2002/0192203 A1 12, 2002 Cho 2003, OOO4194 A1 1, 2003 Gall (73) Assignee: Forest Laboratories Holdings Limited 2003, OO13699 A1 1/2003 Davis et al. (BM) 2003/0027820 A1 2, 2003 Gall (*) Notice: Subject to any disclaimer, the term of this 2003.0053981 A1 3/2003 Davis et al. patent is extended or adjusted under 35 2003, OO60489 A1 3/2003 Buckingham U.S.C. 154(b) by 455 days. 2003, OO69221 A1 4/2003 Kosoglou et al. 2003/0078190 A1* 4/2003 Weinberg ...................... 514f1 (21) Appl. No.: 11/141,235 2003/0078517 A1 4/2003 Kensey 2003/01 19428 A1 6/2003 Davis et al. (22) Filed: May 31, 2005 2003/01 19757 A1 6/2003 Davis 2003/01 19796 A1 6/2003 Strony (65) Prior Publication Data 2003.01.19808 A1 6/2003 LeBeaut et al. US 2005/027281.0 A1 Dec. 8, 2005 2003.01.19809 A1 6/2003 Davis 2003,0162824 A1 8, 2003 Krul Related U.S. Application Data 2003/0175344 A1 9, 2003 Waldet al. (60) Provisional application No. 60/577,423, filed on Jun. -

33-Adrenoceptor-Mediated Relaxation Induced by Isoprenaline And
J. Smooth Muscle Res. 33 : 99-106. 99 The )32 and [33-Adrenoceptor-Mediated Relaxation Induced by Isoprenaline and Salbutamol in Guinea Pig Taenia Caecum Katsuo KOIKE, Tsukasa IcHiNo, Takahiro HORINOUCHI and Issei TAKAYANAGI Departmentof Chemical Pharmacology, Toho University School of PharmaceuticalSciences, 2-2-1, Miyama,Funabashi, Chiba 274, Japan Abstract To understand the receptor subtypes responsible for /3adrenoceptormediated relaxa tion of guinea pig taenia caecum, we investigated the effects of isoprenaline and salbutamol . Isoprenaline and salbutamol caused dose-dependent relaxation of the guinea pig taenia caecum. Propranolol, bupranolol and butoxamine produced shifts of the concentration response curves for isoprenaline and salbutamol. Schild regression analyses carried out for propranolol against isoprenaline and salbutamol gave pA2 values of 8.43 and 8.88, respective ly. Schild regression analyses carried out for butoxamine against isoprenaline and sal butamol gave pA2 values of 6.46 and 6.68, respectively. Schild regression analyses carried out for bupranolol against isoprenaline and salbutamol gave pA2 values of 8.60 and 8.69, respectively. However, in the presence of 3 x 10' M atenolol, 10-4 M butoxamine and 10-6 M phentolamine to block the fir , /32 and a -adrenoceptor effects, respectively, Schild regression analyses carried out for bupranolol against isoprenaline and salbutamol gave pA2 values of 5.77 and 5.97, respectively. These results suggest that the relaxant responses to isoprenaline and salbutamol in the guinea pig taenia caecum are mediated by both the /.32 and the A-adrenoceptors. Key words : f2-adrenoceptor, A-adrenoceptor, isoprenaline, salbutamol , guinea pig taenia caecum Introduction The /3adrenoceptors were subclassified as and /32subtypes based on the agonist potency and tissue localization. -

Relaxation Ofcat Trachea by 13-Adrenoceptor Agonists
Br. J. Pharmac. (1983), 78,417-424 Relaxation of cat trachea by 13-adrenoceptor agonists can be mediated by both f3I- and 132-adrenoceptors and potentiated by inhibitors of extraneuronal uptake Stella R. O'Donnell & Janet C. Wanstall Pharmacology Section, Department of Physiology and Pharmacology, University of Queensland, St Lucia, Brisbane, Qld. 4067, Australia 1 Responses (relaxation) to the P-adrenoceptor agonists, isoprenaline, fenoterol or noradrenaline, were obtained on cat tracheal preparations contracted with a submaximal concentration of carbachol (0.5 JAM). 2 The relative potencies of isoprenaline: fenoterol: noradrenaline were 100:8.1:10.7. From this, it was concluded that responses were mediated predominantly by 131-adrenoceptors but that a minor population of P2-adrenoceptors might also be involved. 3 Schild plots for the selective antagonists atenolol (Pi1-selective) or ICI 118,551 (32-selective) were in different locations, i.e. were separated, depending on whether the antagonist was antagoniz- ing noradrenaline or fenoterol. This supported the conclusion that 12- as well as Pl-adrenoceptors were involved in mediating the response. In this respect, cat trachea resembles cat atria (rate responses). 4 In the presence of atenolol the concentration-response curves to fenoterol became biphasic. This was interpreted as indicating that the P2-adrenoceptors were too few in number to elicit a maximum tissue response. 5 Responses to isoprenaline of cat trachea were potentiated by the extraneuronal uptake inhibitor drugs, corticosterone and metanephrine. This indicated that extraneuronal uptake could modulate P-adrenoceptor-mediated responses (relaxation) of cat trachea. 6 Cat trachea resembles guinea-pig trachea in that (i) the P-adrenoceptor population mediating relaxation is mixed (PI + P2) and (ii) responses to isoprenaline are modulated by its extraneuronal uptake. -

Effects Ofnew Beta-Blocking Agent Wolff-Parkinson-White Syndrome
Br Heart J: first published as 10.1136/hrt.34.12.1272 on 1 December 1972. Downloaded from British Heart Journal, I972, 34) I272-I282. Effects of new beta-blocking agent (DL-tiprenolol) on conduction within normal and anomalous atrioventricular pathways in Wolff-Parkinson-White syndrome J. Roelandt, L. Schamroth, and P. G. Hugenholtz From the Thoraxcentre, University Hospital and Medical Faculty, Rotterdam, The Netherlands; and the Baragwanath Hospital and the University of the Witwatersrand, J7ohannesburg, South Africa The effects of DL-tiprenolol (DU 2I445) - a new beta-receptor blocking agent - and atropine on both normal and abnormal conduction pathways in the Wolff-Parkinson-White (WPW) syn- drome were studied by means of His bundle electrograms and atrial pacing. It was shown that DU 2I445 slows conduction through both pathways significantly (P, o os) and has a potential value in the treatment of symptomatic WPW syndrome and perhaps in other types of re-entrant tachycardias. The effects ofatropine were also studied in 2 patients. Atropine did not affect bypass conduction whereas A V conduction velocity was definitely increased. copyright. The study also shedsfurther light on the mechanism of WPW syndrome. It was found that the bypass had different conducting properties and was most probably a different conducting structure from the normal A V pathway. Surgical division of the anomalous pathway reason for a surgical approach, virtually as a in the Wolff-Parkinson-White (WPW) syn- measure of desperation. http://heart.bmj.com/ drome has recently been carried out with some Little is known about the conduction success in a few patients with incapacitating properties of, and the influence of drugs on, attacks of tachycardia (Cobb et al., I968; the anomalous bypass. -
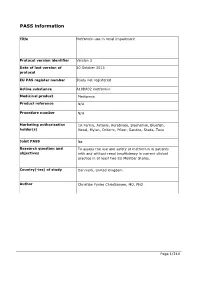
Guidance for the Format and Content of the Protocol of Non-Interventional
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO).