1-(4-Amino-Cyclohexyl)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2012/0190743 A1 Bain Et Al
US 2012O190743A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0190743 A1 Bain et al. (43) Pub. Date: Jul. 26, 2012 (54) COMPOUNDS FOR TREATING DISORDERS Publication Classification OR DISEASES ASSOCATED WITH (51) Int. Cl NEUROKININ 2 RECEPTORACTIVITY A6II 3L/23 (2006.01) (75) Inventors: Jerald Bain, Toronto (CA); Joel CD7C 69/30 (2006.01) Sadavoy, Toronto (CA); Hao Chen, 39t. ii; C Columbia, MD (US); Xiaoyu Shen, ( .01) Columbia, MD (US) A6IPI/00 (2006.01) s A6IP 29/00 (2006.01) (73) Assignee: UNITED PARAGON A6IP II/00 (2006.01) ASSOCIATES INC., Guelph, ON A6IPI3/10 (2006.01) (CA) A6IP 5/00 (2006.01) A6IP 25/00 (2006.01) (21) Appl. No.: 13/394,067 A6IP 25/30 (2006.01) A6IP5/00 (2006.01) (22) PCT Filed: Sep. 7, 2010 A6IP3/00 (2006.01) CI2N 5/071 (2010.01) (86). PCT No.: PCT/US 10/48OO6 CD7C 69/33 (2006.01) S371 (c)(1) (52) U.S. Cl. .......................... 514/552; 554/227; 435/375 (2), (4) Date: Apr. 12, 2012 (57) ABSTRACT Related U.S. Application Data Compounds, pharmaceutical compositions and methods of (60) Provisional application No. 61/240,014, filed on Sep. treating a disorder or disease associated with neurokinin 2 4, 2009. (NK) receptor activity. Patent Application Publication Jul. 26, 2012 Sheet 1 of 12 US 2012/O190743 A1 LU 1750 15OO 1250 OOO 750 500 250 O O 20 3O 40 min SampleName: EM2OO617 Patent Application Publication Jul. 26, 2012 Sheet 2 of 12 US 2012/O190743 A1 kixto CFUgan <tro CFUgan FIG.2 Patent Application Publication Jul. -

A Selective Nociceptin Receptor Antagonist to Treat Depression: Evidence from Preclinical and Clinical Studies
Neuropsychopharmacology (2016) 41, 1803–1812 © 2016 American College of Neuropsychopharmacology. All rights reserved 0893-133X/16 www.neuropsychopharmacology.org A Selective Nociceptin Receptor Antagonist to Treat Depression: Evidence from Preclinical and Clinical Studies ,1 1 2 3 4 Anke Post* , Trevor S Smart , Judith Krikke-Workel , Gerard R Dawson , Catherine J Harmer , 3,4 1 5 6 6 1 Michael Browning , Kimberley Jackson , Rishi Kakar , Richard Mohs , Michael Statnick , Keith Wafford , 1 6 6 Andrew McCarthy , Vanessa Barth and Jeffrey M Witkin 1 2 3 4 5 Lilly UK, Windlesham, Surrey, UK; Eli Lilly, Netherlands; P1vital Limited, Oxfordshire, UK; University of Oxford, Oxford, UK; Innovative Clinical 6 Research-SICR, Ft. Lauderdale, FL, USA; Neuroscience Research, Eli Lilly and Company, Indianapolis, IN, USA Nociceptin/Orphanin FQ (N/OFQ) is an endogenous ligand of the N/OFQ peptide (NOP) receptor, which is a G protein-coupled receptor in brain regions associated with mood disorders. We used a novel, potent, and selective orally bioavailable antagonist, LY2940094, to test the hypothesis that blockade of NOP receptors would induce antidepressant effects. In this study we demonstrate that targeting NOP receptors with LY2940094 translates to antidepressant-like effects in rodent models and, importantly, to antidepressant efficacy in patients with major depressive disorder (MDD). The proof-of-concept study (POC) was an 8-week, double-blind, placebo- controlled trial that evaluated LY2940094 as a novel oral medication for the treatment of patients with MDD. Once daily oral dosing of LY2940094 at 40 mg for 8 weeks vs placebo provided some evidence for an antidepressant effect based on the change from baseline to week 8 in the GRID-Hamilton Depression Rating Scale-17 item total score, although the predefined POC efficacy criterion (probability of ⩾ LY2940094 being better than placebo 88%) was not met (82.9%). -
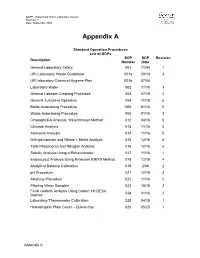
Lab Standard Operating Procedures (Sops)
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Clonazepam/Ethosuximide 479 Abnormal Movements, and for the Treatment of Panic BNFC Suggests Giving the Following Doses by Slow Intravenous 3
Clonazepam/Ethosuximide 479 abnormal movements, and for the treatment of panic BNFC suggests giving the following doses by slow intravenous 3. Peled R, Lavie P. Double-blind evaluation of clonazepam on pe- injection over at least 2 minutes according to age: riodic leg movements in sleep. J Neurol Neurosurg Psychiatry disorder (see Psychiatric Disorders, below). 1987; 50: 1679–81. For epilepsy and myoclonus treatment is started with • neonates: 100 micrograms/kg, repeated if necessary after 24 4. Saletu M, et al. Restless legs syndrome (RLS) and periodic limb hours movement disorder (PLMD): acute placebo-controlled sleep lab- small doses that are progressively increased to an opti- oratory studies with clonazepam. Eur Neuropsychopharmacol • 1 month to 12 years: 50 micrograms/kg (maximum 1 mg), re- 2001; 11: 153–61. mum dose according to response. Total daily doses peated if necessary may initially be taken in 3 or 4 divided doses; however, 5. Saletu A, et al. On the pharmacotherapy of sleep bruxism: place- Older children may be given the usual adult dose. bo-controlled polysomnographic and psychometric studies with once the maintenance dose has been reached, the daily clonazepam. Neuropsychobiology 2005; 51: 214–25. In children aged over 1 month, these doses by injection may be amount may be given as a single dose at night. In the followed by an intravenous infusion of 10 micrograms/kg per Stiff-man syndrome. Clonazepam has been used as an alter- UK the initial oral dose is 1 mg (500 micrograms in the native to diazepam in the management of stiff-man syndrome hour, adjusted according to response to a maximum of 1 elderly) at night for 4 nights gradually increased over 2 60 micrograms/kg per hour. -

Enkephalin Degradation in Serum of Patients with Inflammatory Bowel Diseases
Pharmacological Reports 71 (2019) 42–47 Contents lists available at ScienceDirect Pharmacological Reports journal homepage: www.elsevier.com/locate/pharep Original article Enkephalin degradation in serum of patients with inflammatory bowel diseases a, a b Beata Wilenska *, Dagmara Tymecka , Marcin Włodarczyk , b c Aleksandra Sobolewska-Włodarczyk , Maria Wisniewska-Jarosinska , d e b a,d, Jolanta Dyniewicz , Árpád Somogyi , Jakub Fichna , Aleksandra Misicka * a Faculty of Chemistry, Biological and Chemical Research Centre, University of Warsaw, Warszawa, Poland b Department of Biochemistry, Medical University of Lodz, Łódz, Poland c Department of Gastroenterology, Medical University of Lodz, Łódz, Poland d Department of Neuropeptides, Mossakowski Medical Research Centre Polish Academy of Science, Warszawa, Poland e Campus Chemical Instrumentation Centre (CCIC), The Ohio State University, Columbus, OH, USA A R T I C L E I N F O A B S T R A C T Article history: Background: Inflammatory bowel diseases (IBD) are a group of chronic and recurrent gastrointestinal Received 18 April 2018 disorders that are difficult to control. Recently, a new IBD therapy based on the targeting of the Received in revised form 10 June 2018 endogenous opioid system has been proposed. Consequently, due to the fact that endogenous Accepted 1 August 2018 enkephalins have an anti-inflammatory effect, we aimed at investigating the degradation of serum Available online 2 August 2018 enkephalin (Met- and Leu-enkephalin) in patients with IBD. Methods: Enkephalin degradation in serum of patients with IBD was characterized using mass Keywords: spectrometry methods. Calculated half-life (T1/2) of enkephalins were compared and correlated with the Inflammatory bowel diseases disease type and gender of the patients. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Preventive Report Appendix
Title Authors Published Journal Volume Issue Pages DOI Final Status Exclusion Reason Nasal sumatriptan is effective in treatment of migraine attacks in children: A Ahonen K.; Hamalainen ML.; Rantala H.; 2004 Neurology 62 6 883-7 10.1212/01.wnl.0000115105.05966.a7 Deemed irrelevant in initial screening Seasonal variation in migraine. Alstadhaug KB.; Salvesen R.; Bekkelund SI. Cephalalgia : an 2005 international journal 25 10 811-6 10.1111/j.1468-2982.2005.01018.x Deemed irrelevant in initial screening Flunarizine, a calcium channel blocker: a new prophylactic drug in migraine. Amery WK. 1983 Headache 23 2 70-4 10.1111/j.1526-4610.1983.hed2302070 Deemed irrelevant in initial screening Monoamine oxidase inhibitors in the control of migraine. Anthony M.; Lance JW. Proceedings of the 1970 Australian 7 45-7 Deemed irrelevant in initial screening Prostaglandins and prostaglandin receptor antagonism in migraine. Antonova M. 2013 Danish medical 60 5 B4635 Deemed irrelevant in initial screening Divalproex extended-release in adolescent migraine prophylaxis: results of a Apostol G.; Cady RK.; Laforet GA.; Robieson randomized, double-blind, placebo-controlled study. WZ.; Olson E.; Abi-Saab WM.; Saltarelli M. 2008 Headache 48 7 1012-25 10.1111/j.1526-4610.2008.01081.x Deemed irrelevant in initial screening Divalproex sodium extended-release for the prophylaxis of migraine headache in Apostol G.; Lewis DW.; Laforet GA.; adolescents: results of a stand-alone, long-term open-label safety study. Robieson WZ.; Fugate JM.; Abi-Saab WM.; 2009 Headache 49 1 45-53 10.1111/j.1526-4610.2008.01279.x Deemed irrelevant in initial screening Safety and tolerability of divalproex sodium extended-release in the prophylaxis of Apostol G.; Pakalnis A.; Laforet GA.; migraine headaches: results of an open-label extension trial in adolescents. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

(12) United States Patent (10) Patent No.: US 8,357,723 B2 Satyam (45) Date of Patent: Jan
US008357723B2 (12) United States Patent (10) Patent No.: US 8,357,723 B2 Satyam (45) Date of Patent: Jan. 22, 2013 (54) PRODRUGS CONTAINING NOVEL "Nitric Oxide Donors and Cardiovascular Agents Modulating the BO-CLEAVABLE LINKERS Bioactivity of Nitric Oxide: An Overview” by Louis J. Ignarro, et al., Circulation Research, vol. 90, No. 1, pp. 21-22 (Jan. 11, 002). (75) Inventor: Apparao Satyam, Mumbai (IN) “Bis3-(4-substituted phenyl)prop-2-enedisulfides as a new class of (73) Assignee: Piramal Enterprises Limited and antihyperlipidemic compounds' by Meenakshi Sharma, et al., Apparao Satyam, Mumbai (IN) Bioorganic and Medicinal Chemistry Leters, vol. 14, No. 21, pp. (*) Notice: Subject to any disclaimer, the term of this 5347-5350 (Nov. 1, 2004). patent is extended or adjusted under 35 Abstract Only "Spectrophotometric determination of binary mix U.S.C. 154(b) by 0 days. tures of pseudoephedrine with some histamine H1-receptor antago nists using derivative radio spectrum method' by H. Mahgouh, et al., (21) Appl. No.: 12/977,929 J. Pham Biomed Anal, vol. 31, No. 4, pp. 801-809 Mar. 26, 2003. (22) Filed: Dec. 23, 2010 Peter D. Senter et al., Development of Drug-Release Strategy Based (65) Prior Publication Data on the Reductive Fragmentation pf Benzyl Carbamate Disulfides. Journal of Organic Chemistry, 1990, 55, 2975-2978. Published by US 2011 FO269709 A1 Nov. 3, 2011 American Chemical Society (USA). Related U.S. Application Data Vivekananda M. Virudhula et al., Reductively Activated Disulfide Prodrugs of Paclitaxel. Biorganic & Medicinal Chemistry Letters, (62) Division of application No. 1 1/213,396, filed on Aug.