Supplementary Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endothelin System and Therapeutic Application of Endothelin Receptor
xperim ACCESS Freely available online & E en OPEN l ta a l ic P in h l a C r m f o a c l a o n l o r g u y o J Journal of ISSN: 2161-1459 Clinical & Experimental Pharmacology Research Article Endothelin System and Therapeutic Application of Endothelin Receptor Antagonists Abebe Basazn Mekuria, Zemene Demelash Kifle*, Mohammedbrhan Abdelwuhab Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia ABSTRACT Endothelin is a 21 amino acid molecule endogenous potent vasoconstrictor peptide. Endothelin is synthesized in vascular endothelial and smooth muscle cells, as well as in neural, renal, pulmonic, and inflammatory cells. It acts through a seven transmembrane endothelin receptor A (ETA) and endothelin receptor B (ETB) receptors belongs to G protein-coupled rhodopsin-type receptor superfamily. This peptide involved in pathogenesis of cardiovascular disorder like (heart failure, arterial hypertension, myocardial infraction and atherosclerosis), renal failure, pulmonary arterial hypertension and it also involved in pathogenesis of cancer. Potentially endothelin receptor antagonist helps the treatment of the above disorder. Currently, there are a lot of trails both per-clinical and clinical on endothelin antagonist for various cardiovascular, pulmonary and cancer disorder. Some are approved by FAD for the treatment. These agents are including both selective and non-selective endothelin receptor antagonist (ETA/B). Currently, Bosentan, Ambrisentan, and Macitentan approved -

Crofelemer Oral Delayed Release Tablet
Contains Nonbinding Recommendations Draft – Not for Implementation Draft Guidance on Crofelemer This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Crofelemer Dosage Form; Route: Tablet, delayed release; oral Strength: 125 mg Recommendations for the Assessment of Identity and Quality of Botanical Raw Material (BRM): Crofelemer is a botanical drug derived from BRM, the crude red latex of Croton lechleri Müll. Arg. [Fam. Euphorbiacae], which is also called dragon’s blood (sangre de drago). Generic drug applicants should use the same plant species and perform BRM assessment: 1. Crofelemer BRM should be collected from the crude red latex of Croton lechleri. The plant species should be correctly identified and authenticated based on techniques such as macroscopic/microscopic and/or analysis of genetic material. 2. Crude red latex as BRM should be collected from the mature tree with defined eco- geographic regions (EGRs). Implementing and enforcing established good agricultural and collection practice (GACP) procedures will minimize variations in BRM and ensure batch- to-batch consistency of crofelemer. 3. BRMs should be analyzed for their crofelemer content, total phenolics and taspine content, as well as heavy metals and pesticides. Recommendations for Demonstrating API Sameness: API sameness can be established by showing equivalence between Test API and API from the reference listed drug (RLD) product with the three criteria described in detail below. -
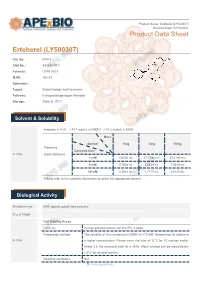
Erteberel (LY500307) Product Data Sheet
Product Name: Erteberel (LY500307) Revision Date: 01/10/2021 Product Data Sheet Erteberel (LY500307) Cat. No.: B1518 CAS No.: 533884-09-2 Formula: C18H18O3 M.Wt: 282.33 Synonyms: Target: Endocrinology and Hormones Pathway: Estrogen/progestogen Receptor Storage: Store at -20°C Solvent & Solubility insoluble in H2O; ≥14.1 mg/mL in DMSO; ≥48.3 mg/mL in EtOH Mass Solvent 1mg 5mg 10mg Preparing Concentration In Vitro Stock Solutions 1 mM 3.5420 mL 17.7098 mL 35.4195 mL 5 mM 0.7084 mL 3.5420 mL 7.0839 mL 10 mM 0.3542 mL 1.7710 mL 3.5420 mL Please refer to the solubility information to select the appropriate solvent. Biological Activity Shortsummary ERβ agonist, potent and selective IC₅₀ & Target Cell Viability Assay Cell Line: Human prostate cancer cell line (PC-3 cells) Preparation method: The solubility of this compound in DMSO is >10 mM. General tips for obtaining In Vitro a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. Reacting conditions: N/A 1 | www.apexbt.com Applications: Erteberel showed potent and selective binding affinity for ERβ with EC50 value of 0.66 nM [1]. Animal experiment Animal models: Male and female rat fertility and rat and rabbit embryo-fetal development model Dosage form: 0.03 to 10 mg/kg/day for rats, or 1 to 25 mg/kg/day for rabbits, oral gavage, for 2 or 10 weeks Applications: There were no-observed adverse effect levels following LY500307 In Vivo administration of 1 mg/kg/day for male rat fertility, 0.3 mg/kg/day for female rat fertility and embryo-fetal development, and 25 mg/kg/day for rabbit embryo-fetal development [2]. -
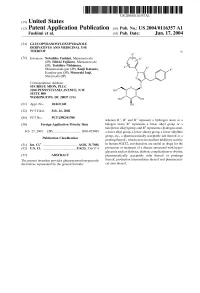
(12) Patent Application Publication (10) Pub. No.: US 2004/0116357 A1 Fushimi Et Al
US 2004O116357A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0116357 A1 Fushimi et al. (43) Pub. Date: Jun. 17, 2004 (54) GLUCOPYRANOSYLOXYPYRAZOLE DERVATIVES AND MEDICINAL USE THEREOF (I) (76) Inventors: Nobuhiko Fushimi, Matsumoto-shi (JP); Hideki Fujikura, Matsumoto-shi (JP); Toshihiro Nishimura, Minamiazumi-gun (JP); Kenji Katsuno, Kamiina-gun (JP); Masayuki Isaji, Shiojiri-shi (JP) Correspondence Address: SUGHRUE MION, PLLC 2100 PENNSYLVANIAAVENUE, N.W. SUTE 800 WASHINGTON, DC 20037 (US) (21) Appl. No.: 10/469,140 (22) PCT Filed: Feb. 26, 2002 (86) PCT No.: PCT/JP02/01708 wherein R', R and R represent a hydrogen atom or a (30) Foreign Application Priority Data halogen atom; R" represents a lower alkyl group or a halo(lower alkyl) group; and R represents a hydrogen atom, Feb. 27, 2001 (JP)...................................... 2001-053085 a lower alkyl group, a lower alkoxy group, a lower alkylthio group, etc., a pharmaceutically acceptable Salt thereof or a Publication Classification prodrug thereof., which exert an excellent inhibitory activity (51) Int. Cl. ................................................ A61K 31/7056 in human SGLT2, and therefore are useful as drugs for the (52) U.S. Cl. ............................................. 514/23: 536/17.4 prevention or treatment of a disease associated with hyper glycemia Such as diabetes, diabetic complications or obesity, (57) ABSTRACT pharmaceutically acceptable Salts thereof or prodrugs The present invention provides glucopyranosyloxypyrazole thereof, production intermediates thereof and pharmaceuti derivatives represented by the general formula: cal uses thereof. US 2004/0116357 A1 Jun. 17, 2004 GLUCOPYRANOSYLOXYPYRAZOLE 0005. In recent years, development of new type antidia DERVATIVES AND MEDICINAL USE THEREOF betic agents has been progressing, which promote urinary glucose excretion and lower blood glucose level by prevent TECHNICAL FIELD ing excess glucose reabsorption at the kidney (J. -

A Multifaceted Approach to Combating Leishmaniasis, a Neglected Tropical Disease
OLD TARGETS AND NEW BEGINNINGS: A MULTIFACETED APPROACH TO COMBATING LEISHMANIASIS, A NEGLECTED TROPICAL DISEASE DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy from the Graduate School of The Ohio State University By Adam Joseph Yakovich, B.S. ***** The Ohio State University 2007 Dissertation Committee: Karl A Werbovetz, Ph.D., Advisor Approved by Pui-Kai Li, Ph.D. Werner Tjarks, Ph.D. ___________________ Ching-Shih Chen, Ph.D Advisor Graduate Program In Pharmacy ABSTRACT Leishmaniasis, a broad spectrum of disease which is caused by the protozoan parasite Leishmania , currently affects 12 million people in 88 countries worldwide. There are over 2 million of new cases of leishmaniasis occurring annually. Clinical manifestations of leishmaniasis range from potentially disfiguring cutaneous leishmaniasis to the most severe manifestation, visceral leishmaniasis, which attacks the reticuloendothelial system and has a fatality rate near 100% if left untreated. All currently available therapies all suffer from drawbacks including expense, route of administration and developing resistance. In the laboratory of Dr. Karl Werbovetz our primary goal is the identification and development of an inexpensive, orally available antileishmanial chemotherapeutic agent. Previous efforts in the lab have identified a series of dinitroaniline compounds which have promising in vitro activity in inhibiting the growth of Leishmania parasites. It has since been discovered that these compounds exert their antileishmanial effects by binding to tubulin and inhibiting polymerization. Remarkably, although mammalian and Leishmania tubulins are ~84 % identical, the dinitroaniline compounds show no effect on mammalian tubulin at concentrations greater than 10-fold the IC 50 value determined for inhibiting Leishmania tubulin ii polymerization. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Targeting Multidrug Resistance Proteins and C-Type Natriuretic Peptide to Optimise Cyclic GMP Signalling in Cardiovascular Disease
Targeting multidrug resistance proteins and C-type natriuretic peptide to optimise cyclic GMP signalling in cardiovascular disease Robert Matthew Henry Grange Submitted in partial fulfilment of the requirements of the Degree of Doctor of Philosophy Heart Centre William Harvey Research Institute Barts & The London School of Medicine & Dentistry Queen Mary University of London Charterhouse Square London EC1M 6BQ United Kingdom STATEMENT OF ORIGINALITY I, Robert Matthew Henry Grange, confirm that the research included within this thesis is my own work or that where it has been carried out in collaboration with, or supported by others, that this is duly acknowledged below and my contribution indicated. Previously published material is also acknowledged below. I attest that I have exercised reasonable care to ensure that the work is original, and does not to the best of my knowledge break any UK law, infringe any third party’s copyright or other Intellectual Property Right, or contain any confidential material. I accept that the College has the right to use plagiarism detection software to check the electronic version of the thesis. I confirm that this thesis has not been previously submitted for the award of a degree by this or any other university. The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without the prior written consent of the author. Signature: Date: 05/05/2016 I PUBLISHED ABSTRACTS Allen RMH, Renukanthan A, Bubb KJ, Villar IC, Moyes AJ, Baliga RS, Hobbs AJ. Investigation of the role of multidrug resistance proteins (MRPs) in vascular homeostasis. -

Computational Drug Target Screening Through Protein Interaction Profiles
www.nature.com/scientificreports OPEN Computational Drug Target Screening through Protein Interaction Profiles Received: 27 June 2016 Santiago Vilar1,2, Elías Quezada3, Eugenio Uriarte2, Stefano Costanzi4, Fernanda Borges3, Accepted: 24 October 2016 Dolores Viña5 & George Hripcsak1 Published: 15 November 2016 The development of computational methods to discover novel drug-target interactions on a large scale is of great interest. We propose a new method for virtual screening based on protein interaction profile similarity to discover new targets for molecules, including existing drugs. We calculated Target Interaction Profile Fingerprints (TIPFs) based on ChEMBL database to evaluate drug similarity and generated new putative compound-target candidates from the non-intersecting targets in each pair of compounds. A set of drugs was further studied in monoamine oxidase B (MAO-B) and cyclooxygenase-1 (COX-1) enzyme through molecular docking and experimental assays. The drug ethoxzolamide and the natural compound piperlongumine, present in Piper longum L, showed hMAO-B activity with IC50 values of 25 and 65 μM respectively. Five candidates, including lapatinib, SB-202190, RO-316233, GW786460X and indirubin-3′-monoxime were tested against human COX-1. Compounds SB-202190 and RO-316233 showed a IC50 in hCOX-1 of 24 and 25 μM respectively (similar range as potent inhibitors such as diclofenac and indomethacin in the same experimental conditions). Lapatinib and indirubin- 3′-monoxime showed moderate hCOX-1 activity (19.5% and 28% of enzyme inhibition at 25 μM respectively). Our modeling constitutes a multi-target predictor for large scale virtual screening with potential in lead discovery, repositioning and drug safety. Discovery of new targets for molecules is of great interest in drug design and development1,2. -

WHO Drug Information Vol
WHO Drug Information Vol. 24, No. 4, 2010 World Health Organization WHO Drug Information Contents WHO Prequalification Sitaxentan: worldwide withdrawal 307 Programmes Sibutramine: suspension of sales 307 Sibutramine-containing medicines: WHO Prequalification of Medicines withdrawal 308 Programme: survey of service Testosterone transdermal patch: quality provided to manufacturers 293 withdrawal of extension of WHO initiates pilot prequalification of indication application 308 active pharmaceutical ingredients 297 Aliskiren/valsartan: withdrawal of New on-line database for WHO marketing authorization application 308 prequalified vaccines 298 Mometasone furoate/formoterol fumarate: withdrawal of marketing Safety and Efficacy Issues authorization application 309 EMA and US FDA extend confidentiality H1N1 influenza vaccine: narcolepsy 299 arrangements indefinitely 309 Statins: interstitial lung disease 299 Tocilizumab: risk of fatal anaphylaxis 300 Recent Publications, Pioglitazone: potential bladder cancer 301 Information and Events Angiotensin receptor blockers and US Government to share patents with cancer: safety review 301 Medicines Patent Pool 310 GnRH agonists, diabetes and cardio- Clinical trials and global medicines vascular disease 301 development 310 Gadolinium-based contrast agents: Evaluation of future nanomedicines 311 kidney dysfunction 302 Reporting on opioid inaccessibility 311 Lamotrigine: aseptic meningitis Tinzaparin sodium: renal Impairment in elderly 303 Consultation Documents Tamoxifen: drug interactions involving The -

Drug Class Review Ophthalmic Cholinergic Agonists
Drug Class Review Ophthalmic Cholinergic Agonists 52:40.20 Miotics Acetylcholine (Miochol-E) Carbachol (Isopto Carbachol; Miostat) Pilocarpine (Isopto Carpine; Pilopine HS) Final Report November 2015 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Carin Steinvoort, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor University of Utah College of Pharmacy Copyright © 2015 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved. Table of Contents Executive Summary ......................................................................................................................... 3 Introduction .................................................................................................................................... 4 Table 1. Glaucoma Therapies ................................................................................................. 5 Table 2. Summary of Agents .................................................................................................. 6 Disease Overview ........................................................................................................................ 8 Table 3. Summary of Current Glaucoma Clinical Practice Guidelines ................................... 9 Pharmacology ............................................................................................................................... 10 Methods ....................................................................................................................................... -

Lung Inflammation and Atopy Muscarinic Receptor Expression
The Journal of Immunology Maternal Exposure to Secondhand Cigarette Smoke Primes the Lung for Induction of Phosphodiesterase-4D5 Isozyme and Exacerbated Th2 Responses: Rolipram Attenuates the Airway Hyperreactivity and Muscarinic Receptor Expression but Not Lung Inflammation and Atopy1 Shashi P. Singh,* Neerad C. Mishra,* Jules Rir-sima-ah,* Mathew Campen,* Viswanath Kurup,† Seddigheh Razani-Boroujerdi,* and Mohan L. Sopori2* Airway hyperreactivity (AHR), lung inflammation, and atopy are clinical signs of allergic asthma. Gestational exposure to ciga- rette smoke (CS) markedly increases the risk for childhood allergic asthma. Muscarinic receptors regulate airway smooth muscle tone, and asthmatics exhibit increased AHR to muscarinic agonists. We have previously reported that in a murine model of bronchopulmonary aspergillosis, maternal exposure to mainstream CS increases AHR after acute intratracheal administration of Aspergillus fumigatus extract. However, the mechanism by which gestational CS induces allergic asthma is unclear. We now show for the first time that, compared with controls, mice exposed prenatally to secondhand CS exhibit increased lung inflammation (predominant infiltration by eosinophils and polymorphs), atopy, and airway resistance, and produce proinflammatory cytokines (IL-4, IL-5, IL-6, and IL-13, but not IL-2 or IFN-␥). These changes, which occur only after an allergen (A. fumigatus extract) treatment, are correlated with marked up-regulated lung expression of M1, M2, and M3 muscarinic receptors and phosphodi- esterase (PDE)4D5 isozyme. Interestingly, the PDE4-selective inhibitor rolipram attenuates the increase in AHR, muscarinic receptors, and PDE4D5, but fails to down-regulate lung inflammation, Th2 cytokines, or serum IgE levels. Thus, the fetus is extraordinarily sensitive to CS, inducing allergic asthma after postnatal exposure to allergens. -

2-Amino-1,3,4-Thiadiazoles in Leishmaniasis
Review Future Prospects in the Treatment of Parasitic Diseases: 2‐Amino‐1,3,4‐Thiadiazoles in Leishmaniasis Georgeta Serban Pharmaceutical Chemistry Department, Faculty of Medicine and Pharmacy, University of Oradea, 29 Nicolae Jiga, 410028 Oradea, Romania; [email protected]; Tel: +4‐0756‐276‐377 Received: 22 March 2019; Accepted: 17 April 2019; Published: 19 April 2019 Abstract: Neglected tropical diseases affect the lives of a billion people worldwide. Among them, the parasitic infections caused by protozoan parasites of the Trypanosomatidae family have a huge impact on human health. Leishmaniasis, caused by Leishmania spp., is an endemic parasitic disease in over 88 countries and is closely associated with poverty. Although significant advances have been made in the treatment of leishmaniasis over the last decade, currently available chemotherapy is far from satisfactory. The lack of an approved vaccine, effective medication and significant drug resistance worldwide had led to considerable interest in discovering new, inexpensive, efficient and safe antileishmanial agents. 1,3,4‐Thiadiazole rings are found in biologically active natural products and medicinally important synthetic compounds. The thiadiazole ring exhibits several specific properties: it is a bioisostere of pyrimidine or benzene rings with prevalence in biologically active compounds; the sulfur atom increases lipophilicity and combined with the mesoionic character of thiadiazoles imparts good oral absorption and good cell permeability, resulting in good bioavailability. This review presents synthetic 2‐amino‐1,3,4‐thiadiazole derivatives with antileishmanial activity. Many reported derivatives can be considered as lead compounds for the synthesis of future agents as an alternative to the treatment of leishmaniasis. Keywords: 2‐amino‐1,3,4‐thiadiazole; neglected tropical diseases; protozoan parasites; Leishmania spp.; antileishmanial activity; inhibitory concentration 1.