TE INI (19 ) United States (12 ) Patent Application Publication ( 10) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of a Test That Measures Real-Time HER2 Signaling Function in Live Breast Cancer Cell Lines and Primary Cells Yao Huang1, David J
Huang et al. BMC Cancer (2017) 17:199 DOI 10.1186/s12885-017-3181-0 RESEARCH ARTICLE Open Access Development of a test that measures real-time HER2 signaling function in live breast cancer cell lines and primary cells Yao Huang1, David J. Burns1, Benjamin E. Rich1, Ian A. MacNeil1, Abhijit Dandapat1, Sajjad M. Soltani1, Samantha Myhre1, Brian F. Sullivan1, Carol A. Lange2, Leo T. Furcht3 and Lance G. Laing1* Abstract Background: Approximately 18–20% of all human breast cancers have overexpressed human epidermal growth factor receptor 2 (HER2). Standard clinical practice is to treat only overexpressed HER2 (HER2+) cancers with targeted anti-HER2 therapies. However, recent analyses of clinical trial data have found evidence that HER2-targeted therapies may benefit a sub-group of breast cancer patients with non-overexpressed HER2. This suggests that measurement of other biological factors associated with HER2 cancer, such as HER2 signaling pathway activity, should be considered as an alternative means of identifying patients eligible for HER2 therapies. Methods: A new biosensor-based test (CELxTM HSF) that measures HER2 signaling activity in live cells is demonstrated using a set of 19 human HER2+ and HER2– breast cancer reference cell lines and primary cell samples derived from two fresh patient tumor specimens. Pathway signaling is elucidated by use of highly specific agonists and antagonists. The test method relies upon well-established phenotypic, adhesion-related, impedance changes detected by the biosensor. Results: The analytical sensitivity and analyte specificity of this method was demonstrated using ligands with high affinity and specificity for HER1 and HER3. The HER2-driven signaling quantified ranged 50-fold between the lowest and highest cell lines. -

Cell Line Descriptions Geneblazer® M1-NFAT
Version No.: GeneBLAzer ® Validation Packet Page 1 of 5 01Sep08 Optimization of the GeneBLazer ® M1-NFAT-bla Jurkat Cell Line GeneBLAzer ® M1 NFAT-bla Jurkat Cells Catalog Numbers – K1710 Cell Line Descriptions GeneBLAzer ® M1-NFAT-bla Jurkat cells contain the human Acetylcholine (muscarinic) subtype 1 receptor (M1), (Accession # NM_000738 ) stably integrated into the CellSensor ® NFAT-bla Jurkat cell line. CellSensor ® NFAT-bla Jurkat cells (Cat. no. K1671) contain a beta-lactamase (bla ) reporter gene under control of the Nuclear Factor of Activated T-cells (NFAT) response element. M1-NFAT-bla Jurkat cells are functionally validated for Z’-factor and EC 50 concentrations of carbachol (Figure 1). In addition, GeneBLAzer ® M1-NFAT-bla CHO-K1 cells have been tested for assay performance under variable conditions. Target Description Muscarinic acetylcholine receptors are members of the G protein-coupled receptor (GPCR) superfamily. Muscarinic receptors are widely distributed and mediate the actions of acetylcholine in both the CNS and peripheral tissues. Five muscarinic receptor subtypes have been identified and are referred to as M 1-M5 (1-5). The five genes that encode the muscarinic receptors all belong to the rhodopsin-line family (Family A) and share strong sequence homology but have unique regions located at the amino terminus (extracellular) and in the third intracellular loop. The M 1, M3, and M 5 receptor subtypes couple through the G q/11 class of G-proteins and activate the phopholipase C pathway. Activation of this pathway in turn leads to increases in free intracellular calcium levels as inositol triphosphate mediates release of calcium from the endoplasmic reticulum. -

Optumrx Brand Pipeline Forecast
RxOutlook® 1st Quarter 2019 OptumRx brand pipeline forecast Route of Regulatory Estimated Specialty Orphan Drug name Generic name Company Drug class Therapeutic use administration status release date drug drug 2019 Possible launch date Ophthalmological DS-300 DS-300 Eton undisclosed SC Filed NDA 2019 unknown N disease anti-sclerostin Evenity romosozumab Amgen Osteoporosis SC Filed NDA 2/2019 Y N monoclonal antibody tetrahydrofolate iclaprim iclaprim Motif Bio Bacterial infections IV Filed NDA 2/13/2019 Y Y dehydrogenase inhibitor tazarotene/ IDP-118 Valeant retinoid/ corticosteroid Psoriasis TOP Filed NDA 2/15/2019 N N halobetasol adenosine deaminase Mavenclad cladribine Merck/ Teva resistant Multiple sclerosis PO Filed NDA 2/15/2019 Y N deoxyadenosine analog Lotemax Gel loteprednol Valeant corticosteroid Ocular inflammation OP Filed NDA 2/25/2019 N N Nex Gen etabonate turoctocog alfa glyco-PEGylated factor NN-7088 Novo Nordisk Hemophilia IV/SC Filed BLA 2/27/2019 Y N pegol VIII derivative selective sphingosine-1 BAF-312 siponimod Novartis phosphate receptor Multiple sclerosis PO Filed NDA 3/1/2019 Y N agonist midazolam midazolam UCB benzodiazepine Seizures Intranasal Filed NDA 3/1/2019 N Y (USL-261) XeriSol glucagon Xeris glucagon analog Diabetes mellitus SC Filed NDA 3/1/2019 N N Glucagon optum.com/optumrx 1 RxOutlook® 1st Quarter 2019 Route of Regulatory Estimated Specialty Orphan Drug name Generic name Company Drug class Therapeutic use administration status release date drug drug dopamine receptor JZP-507 sodium oxybate Jazz Narcolepsy -

Phase II Study of Hormone Therapy with Tamoxifen in Patients with Well Differentiated Neuroendocrine Tumors and Hormone Receptor Positive Expression (HORMONET)
Phase II study of hormone therapy with tamoxifen in patients with well differentiated neuroendocrine tumors and hormone receptor positive expression (HORMONET) Time of researchers and respective Departments: Rachel Riechelmann (Main Investigador)1, Milton Barros 1, Marcos Camandaroba1,2, Virgilio Souza1,2, Celso Abdon Mello1, Paula Nicole3, Eduardo Nóbrega4, Ludmilla Chinen5, Marina De Brot6, Héber Salvador7. Departaments: 1- Clinical Oncology; 2- postgraduate student; 3- Radiology; 4- Nuclear Medicine; 5-International Research Center (CIPE); 6-Pathologic anatomy; 7- Abdomen Surgery AC Camargo Cancer Center - Brazil Registration Number on Institutional Research Ethics Committee: 2626/18 March,06,2019 Introduction Neuroendocrine tumors (NET) are rare neoplasms, but with increasing incidence and prevalence in the last decades. Although they may manifest in the most diverse tissues, the vast majority of cases will affect organs of the digestive tract and lung. At diagnosis, more than half of the cases present metastatic disease, and among patients with localized disease, up to one-third will have recurrence of the disease. Unfortunately, the minority of patients with metastatic disease are eligible for curative intent.1 Although there are many types of NET, they are often studied together as a group because their cells share common histological findings, have special secretory granules, and the ability to secrete bioactive amines and polypeptide hormones. Approximately 25 percent of the tumors present functional hormonal syndromes (situation of great morbidity for these patients), being the carcinoid syndrome, the most common one. From the molecular point of view, these neoplasias are largely dependent on the activation of the mTOR pathway and neoangiogenesis.2 Another striking feature of neuroendocrine cells is the expression of cell surface hormone receptors whose activation or blockade may exert an important regulatory function. -
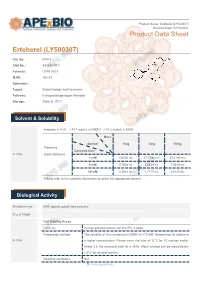
Erteberel (LY500307) Product Data Sheet
Product Name: Erteberel (LY500307) Revision Date: 01/10/2021 Product Data Sheet Erteberel (LY500307) Cat. No.: B1518 CAS No.: 533884-09-2 Formula: C18H18O3 M.Wt: 282.33 Synonyms: Target: Endocrinology and Hormones Pathway: Estrogen/progestogen Receptor Storage: Store at -20°C Solvent & Solubility insoluble in H2O; ≥14.1 mg/mL in DMSO; ≥48.3 mg/mL in EtOH Mass Solvent 1mg 5mg 10mg Preparing Concentration In Vitro Stock Solutions 1 mM 3.5420 mL 17.7098 mL 35.4195 mL 5 mM 0.7084 mL 3.5420 mL 7.0839 mL 10 mM 0.3542 mL 1.7710 mL 3.5420 mL Please refer to the solubility information to select the appropriate solvent. Biological Activity Shortsummary ERβ agonist, potent and selective IC₅₀ & Target Cell Viability Assay Cell Line: Human prostate cancer cell line (PC-3 cells) Preparation method: The solubility of this compound in DMSO is >10 mM. General tips for obtaining In Vitro a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. Reacting conditions: N/A 1 | www.apexbt.com Applications: Erteberel showed potent and selective binding affinity for ERβ with EC50 value of 0.66 nM [1]. Animal experiment Animal models: Male and female rat fertility and rat and rabbit embryo-fetal development model Dosage form: 0.03 to 10 mg/kg/day for rats, or 1 to 25 mg/kg/day for rabbits, oral gavage, for 2 or 10 weeks Applications: There were no-observed adverse effect levels following LY500307 In Vivo administration of 1 mg/kg/day for male rat fertility, 0.3 mg/kg/day for female rat fertility and embryo-fetal development, and 25 mg/kg/day for rabbit embryo-fetal development [2]. -

BMC Pharmacology Biomed Central
CORE Metadata, citation and similar papers at core.ac.uk Provided by Springer - Publisher Connector BMC Pharmacology BioMed Central Research article Open Access Pharmacological Properties of DOV 315,090, an ocinaplon metabolite Dmytro Berezhnoy†1, Maria C Gravielle†1, Scott Downing1, Emmanuel Kostakis1, Anthony S Basile2, Phil Skolnick2, Terrell T Gibbs1 and David H Farb*1 Address: 1Laboratory of Molecular Neurobiology, Department of Pharmacology & Experimental Therapeutics, Boston University School of Medicine, 715 Albany St., Boston, MA 02118, USA and 2DOV Pharmaceutical, Inc, 150 Pierce St., Somerset, NJ 08873-4185, USA Email: Dmytro Berezhnoy - [email protected]; Maria C Gravielle - [email protected]; Scott Downing - [email protected]; Emmanuel Kostakis - [email protected]; Anthony S Basile - [email protected]; Phil Skolnick - [email protected]; Terrell T Gibbs - [email protected]; David H Farb* - [email protected] * Corresponding author †Equal contributors Published: 13 June 2008 Received: 20 December 2007 Accepted: 13 June 2008 BMC Pharmacology 2008, 8:11 doi:10.1186/1471-2210-8-11 This article is available from: http://www.biomedcentral.com/1471-2210/8/11 © 2008 Berezhnoy et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background: Compounds targeting the benzodiazepine binding site of the GABAA-R are widely prescribed for the treatment of anxiety disorders, epilepsy, and insomnia as well as for pre- anesthetic sedation and muscle relaxation. It has been hypothesized that these various pharmacological effects are mediated by different GABAA-R subtypes. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110449 A1 Lorber Et Al
US 200601 10449A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110449 A1 LOrber et al. (43) Pub. Date: May 25, 2006 (54) PHARMACEUTICAL COMPOSITION Publication Classification (76) Inventors: Richard R. Lorber, Scotch Plains, NJ (US); Heribert W. Staudinger, Union, (51) Int. Cl. NJ (US); Robert E. Ward, Summit, NJ A6II 3/473 (2006.01) (US) A6II 3L/24 (2006.01) Correspondence Address: A6IR 9/20 (2006.01) SCHERING-PLOUGH CORPORATION (52) U.S. Cl. ........................... 424/464; 514/290: 514/540 PATENT DEPARTMENT (K-6-1, 1990) 2000 GALLOPNG HILL ROAD KENILWORTH, NJ 07033-0530 (US) (57) ABSTRACT (21) Appl. No.: 11/257,348 (22) Filed: Oct. 24, 2005 The present invention relates to formulations useful for Related U.S. Application Data treating respiratory disorders associated with the production of mucus glycoprotein, skin disorders, and allergic conjunc (60) Provisional application No. 60/622,507, filed on Oct. tivitis while substantially reducing adverse effects associ 27, 2004. Provisional application No. 60/621,783, ated with the administration of non-selective anti-cholin filed on Oct. 25, 2004. ergic agents and methods of use thereof. US 2006/01 10449 A1 May 25, 2006 PHARMACEUTICAL COMPOSITION example, Weinstein and Weinstein (U.S. Pat. No. 6,086,914) describe methods of treating allergic rhinitis using an anti CROSS REFERENCE TO RELATED cholinergic agent with a limited capacity to pass across lipid APPLICATION membranes, such as the blood-brain barrier, in combination with an antihistamine that is limited in both sedating and 0001. This application claims benefit of priority to U.S. -

Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix
United States International Trade Commission Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix USITC Publication 4208 December 2010 U.S. International Trade Commission COMMISSIONERS Deanna Tanner Okun, Chairman Irving A. Williamson, Vice Chairman Charlotte R. Lane Daniel R. Pearson Shara L. Aranoff Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix Publication 4208 December 2010 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 15, 2010, set forth at the end of this publication, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the United States International Trade Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement changes to the Pharmaceutical Appendix, effective on January 1, 2011. Table 1 International Nonproprietary Name (INN) products proposed for addition to the Pharmaceutical Appendix to the Harmonized Tariff Schedule INN CAS Number Abagovomab 792921-10-9 Aclidinium Bromide 320345-99-1 Aderbasib 791828-58-5 Adipiplon 840486-93-3 Adoprazine 222551-17-9 Afimoxifene 68392-35-8 Aflibercept 862111-32-8 Agatolimod -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

Hyperaldosteronism: How Current Concepts Are Transforming the Diagnostic and Therapeutic Paradigm
Kidney360 Publish Ahead of Print, published on July 23, 2020 as doi:10.34067/KID.0000922020 Hyperaldosteronism: How Current Concepts Are Transforming the Diagnostic and Therapeutic Paradigm Michael R Lattanzio(1), Matthew R Weir(2) (1) Division of Nephrology, Department of Medicine, The Chester County Hospital/University of Pennsylvania Health System (2) Division of Nephrology, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD Correspondence: Matthew R Weir University of Maryland Medical Center Division of Nephrology 22 S. Greene St. Room N3W143 Baltimore, Maryland 21201 [email protected] 1 Copyright 2020 by American Society of Nephrology. Abbreviations PA=Primary Hyperaldosteronism CVD=Cardiovascular Disease PAPY=Primary Aldosteronism Prevalence in Hypertension APA=Aldosterone-Producing Adenoma BAH=Bilateral Adrenal Hyperplasia ARR=Aldosterone Renin Ratio AF=Atrial Fibrillation OSA=Obstructive Sleep Apnea OR=Odds Ratio AHI=Apnea Hypopnea Index ABP=Ambulatory Blood Pressure AVS=Adrenal Vein Sampling CT=Computerized Tomography MRI=Magnetic Resonance Imaging SIT=Sodium Infusion Test FST=Fludrocortisone Suppression Test CCT=Captopril Challenge Test PAC=Plasma Aldosterone Concentration PRA=Plasma Renin Activity MRA=Mineralocorticoid Receptor Antagonist MR=Mineralocorticoid Receptor 2 Abstract Nearly seven decades have elapsed since the clinical and biochemical features of Primary Hyperaldosteronism (PA) were described by Conn. PA is now widely recognized as the most common form of secondary hypertension. PA has a strong correlation with cardiovascular disease and failure to recognize and/or properly diagnose this condition has profound health consequences. With proper identification and management, PA has the potential to be surgically cured in a proportion of affected individuals. The diagnostic pursuit for PA is not a simplistic endeavor, particularly since an enhanced understanding of the disease process is continually redefining the diagnostic and treatment algorithm. -

A Simple Method to Measure Sulfonation in Man Using Paracetamol As Probe Drug Natália Marto 1,2*, Judit Morello1,3, Alexandra M
www.nature.com/scientificreports OPEN A simple method to measure sulfonation in man using paracetamol as probe drug Natália Marto 1,2*, Judit Morello1,3, Alexandra M. M. Antunes3, Sofa Azeredo4, Emília C. Monteiro1,5 & Sofa A. Pereira1,5 Sulfotransferase enzymes (SULT) catalyse sulfoconjugation of drugs, as well as endogenous mediators, gut microbiota metabolites and environmental xenobiotics. To address the limited evidence on sulfonation activity from clinical research, we developed a clinical metabolic phenotyping method using paracetamol as a probe substrate. Our aim was to estimate sulfonation capability of phenolic compounds and study its intraindividual variability in man. A total of 36 healthy adult volunteers (12 men, 12 women and 12 women on oral contraceptives) received paracetamol in a 1 g-tablet formulation on three separate occasions. Paracetamol and its metabolites were measured in plasma and spot urine samples using liquid chromatography-high resolution mass spectrometry. A metabolic ratio (Paracetamol Sulfonation Index—PSI) was used to estimate phenol SULT activity. PSI showed low intraindividual variability, with a good correlation between values in plasma and spot urine samples. Urinary PSI was independent of factors not related to SULT activity, such as urine pH or eGFR. Gender and oral contraceptive intake had no impact on PSI. Our SULT phenotyping method is a simple non-invasive procedure requiring urine spot samples, using the safe and convenient drug paracetamol as a probe substrate, and with low intraindividual coefcient of variation. Although it will not give us mechanistic information, it will provide us an empirical measure of an individual’s sulfonator status. To the best of our knowledge, our method provides the frst standardised in vivo empirical measure of an individual’s phenol sulfonation capability and of its intraindividual variability. -

Forendo Pharma Announces the US Licensing of Fispemifene to Apricus Biosciences Targeting Urological Conditions in Men
Forendo Pharma announces the US licensing of fispemifene to Apricus Biosciences targeting urological conditions in men STOCKHOLM, October 20, 2014. Forendo Pharma Oy, a Karolinska Development AB portfolio company, announced today that it has entered into a definitive agreement to out- license the US development and commercialization rights for fispemifene to Apricus Biosciences Inc. Forendo is entitled to success driven milestone payments totaling up to of $305 million plus sales royalties. Karolinska Development has an ownership of 21 percent in Forendo Pharma. Under the terms of the agreement, Apricus will make a $5 million upfront cash payment to Forendo, and will transfer approximately 3.6 million Apricus common shares, representing $7.5 million in value based on the 360-day average market price of the Apricus stock. The agreement includes additional potential clinical and regulatory milestones payments to Forendo for up to $45 million, including FDA approval, as well as commercial milestone payments totaling up to $260 million based on achieving specified annual net sales of fispemifene levels up to $1 billion in the US. Apricus will also pay tiered double-digit royalties based on net sales once the product is commercialized. Apricus will be responsible for the clinical development and costs of the program, as well as all future commercialization in the US. Apricus anticipates to commence a Phase IIb clinical trial during the first half of 2015 to confirm the optimal fispemifene doses to treat men with secondary hypogonadism, and provide proof-of-concept data to evaluate the anti-estrogenic and anti-inflammatory effects on the lower urinary tract and prostate in aging men.