Summary of Product Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Valturna Label
HIGHLIGHTS OF PRESCRIBING INFORMATION -------------------------WARNINGS AND PRECAUTIONS---------------- These highlights do not include all the information needed to use • Avoid fetal or neonatal exposure. (5.1) Valturna safely and effectively. See full prescribing information for • Head and neck angioedema: Discontinue Valturna and monitor until Valturna. signs and symptoms resolve. (5.2) • Hypotension in volume- or salt-depleted Patients: Correct imbalances Valturna (aliskiren and valsartan, USP) Tablets before initiating therapy with Valturna. (5.3) Initial U.S. Approval: 2009 • Patients with renal impairment: Decreases in renal function may be anticipated in susceptible individuals. (5.4) WARNING: AVOID USE IN PREGNANCY • Patients with hepatic impairment: Slower clearance may occur. (5.5) See full prescribing information for complete boxed warning. • Hyperkalemia: Consider periodic determinations of serum electrolytes to When pregnancy is detected, discontinue Valturna as soon as possible. detect possible electrolyte imbalances, particularly in patients at risk. When used in pregnancy during the second and third trimester, drugs (5.7) that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1) --------------------------------ADVERSE REACTIONS----------------------- The most common adverse events (incidence ≥1.5% and more common than ---------------------------INDICATIONS AND USAGE----------------------- with placebo) are: fatigue and nasopharyngitis. (6.1) Valturna is a combination of aliskiren, a direct renin inhibitor, and valsartan, an angiotensin II receptor blocker (ARB), indicated for the treatment of To report SUSPECTED ADVERSE REACTIONS, contact Novartis hypertension: Pharmaceuticals Corporation at 1-888-669-6682 or FDA at • In patients not adequately controlled with monotherapy. (1) 1-800-FDA-1088 or www.fda.gov/medwatch • May be substituted for titrated components. -

Patient Information Telmisartan (TEL-Mi-SAR-Tan) Tablets, USP
Patient Information Telmisartan (TEL-mi-SAR-tan) Tablets, USP Read this Patient Information before you start taking telmisartan tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment. What is the most important information I should know about telmisartan tablets? Telmisartan tablets can cause harm or death to an unborn baby. Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant. If you get pregnant while taking telmisartan tablets, tell your doctor right away. What are telmisartan tablets? Telmisartan tablets are a prescription medicine used: • to treat high blood pressure (hypertension) It is not known if telmisartan tablets are safe and effective in children. Who should not take telmisartan tablets? You should not take telmisartan tablets if you are allergic (hypersensitive) to the active ingredient (telmisartan) or any of the other ingredients listed at the end of this leaflet. For patients with diabetes, if you are taking telmisartan tablets you should not take aliskiren. What should I tell my doctor before taking telmisartan tablets? Before you take telmisartan tablets, tell your doctor if you: • are pregnant or are planning to become pregnant. See “What is the most important information I should know about telmisartan tablets?” • are breast-feeding or plan to breast-feed. It is not known if telmisartan passes into your breast milk. You and your doctor should decide if you will take telmisartan tablets or breast-feed. You should not do both. -

Apo-Cilazapril/Hydrochlorothiazide Film Coated Tablet
New Zealand Data Sheet APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE 1. PRODUCT NAME APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE – cilazapril 5mg and hydrochlorothiazide 12.5mg film coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Cilazapril monohydrate 5.22mg (equivalent to Cilazapril 5mg) and Hydrochlorothiazide 12.5mg Excipient(s) with known effect HYDROCHLOROTHIAZIDE contains sulphur. APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is lactose free and gluten free. APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE contains Red Ferric Oxide (orange shade # 34690). For the full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE are pink, oval biconvex film-coated tablets. Each tablet is engraved “APO” on one side and “5” bisect “12.5” on the other side. Each tablet typically weighs 92mg. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is indicated for the treatment of patients with hypertension who are not adequately controlled on monotherapy. 4.2 Dose and method of administration Standard Dosage The dosage of APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is one tablet administered once daily. As food intake has no clinically significant influence on absorption, APO- CILAZAPRIL/HYDROCHLOROTHIAZIDE can be administered before or after meals. The dose should always be taken at about the same time of day. Special Populations Renal insufficiency When concomitant diuretic therapy is required in patients with severe renal impairment, a loop diuretic rather than a thiazide diuretic is preferred for use with cilazapril/hydrochlorothiazide; therefore, for patients with severe renal dysfunction (creatinine Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 1 of 22 APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE clearance <10ml/min), APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is not recommended. -

Effect of Aldosterone Breakthrough on Albuminuria During Treatment with a Direct Renin Inhibitor and Combined Effect with a Mineralocorticoid Receptor Antagonist
Hypertension Research (2013) 36, 879–884 & 2013 The Japanese Society of Hypertension All rights reserved 0916-9636/13 www.nature.com/hr ORIGINAL ARTICLE Effect of aldosterone breakthrough on albuminuria during treatment with a direct renin inhibitor and combined effect with a mineralocorticoid receptor antagonist Atsuhisa Sato and Seiichi Fukuda We have reported observing aldosterone breakthrough in the course of relatively long-term treatment with renin–angiotensin (RA) system inhibitors, where the plasma aldosterone concentration (PAC) increased following an initial decrease. Aldosterone breakthrough has the potential to eliminate the organ-protective effects of RA system inhibitors. We therefore conducted a study in essential hypertensive patients to determine whether aldosterone breakthrough occurred during treatment with the direct renin inhibitor (DRI) aliskiren and to ascertain its clinical significance. The study included 40 essential hypertensive patients (18 men and 22 women) who had been treated for 12 months with aliskiren. Aliskiren significantly decreased blood pressure and plasma renin activity (PRA). The PAC was also decreased significantly at 3 and 6 months; however, the significant difference disappeared after 12 months. Aldosterone breakthrough was observed in 22 of the subjects (55%). Urinary albumin excretion differed depending on whether breakthrough occurred. For the subjects in whom aldosterone breakthrough was observed, eplerenone was added. A significant decrease in urinary albumin excretion was observed after 1 month, independent of changes in blood pressure. In conclusion, this study demonstrated that aldosterone breakthrough occurs in some patients undergoing DRI therapy. Aldosterone breakthrough affects the drug’s ability to improve urinary albumin excretion, and combining a mineralocorticoid receptor antagonist with the DRI may be useful for decreasing urinary albumin excretion. -

A Comparison of the Tolerability of the Direct Renin Inhibitor Aliskiren and Lisinopril in Patients with Severe Hypertension
Journal of Human Hypertension (2007) 21, 780–787 & 2007 Nature Publishing Group All rights reserved 0950-9240/07 $30.00 www.nature.com/jhh ORIGINAL ARTICLE A comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertension RH Strasser1, JG Puig2, C Farsang3, M Croket4,JLi5 and H van Ingen4 1Technical University Dresden, Heart Center, University Hospital, Dresden, Germany; 2Department of Internal Medicine, La Paz Hospital, Madrid, Spain; 31st Department of Internal Medicine, Semmelweis University, Budapest, Hungary; 4Novartis Pharma AG, Basel, Switzerland and 5Novartis Institutes for Biomedical Research, Cambridge, MA, USA Patients with severe hypertension (4180/110 mm Hg) LIS 3.4%). The most frequently reported AEs in both require large blood pressure (BP) reductions to reach groups were headache, nasopharyngitis and dizziness. recommended treatment goals (o140/90 mm Hg) and At end point, ALI showed similar mean reductions from usually require combination therapy to do so. This baseline to LIS in msDBP (ALI À18.5 mm Hg vs LIS 8-week, multicenter, randomized, double-blind, parallel- À20.1 mm Hg; mean treatment difference 1.7 mm Hg group study compared the tolerability and antihyperten- (95% confidence interval (CI) À1.0, 4.4)) and mean sitting sive efficacy of the novel direct renin inhibitor aliskiren systolic blood pressure (ALI À20.0 mm Hg vs LIS with the angiotensin converting enzyme inhibitor À22.3 mm Hg; mean treatment difference 2.8 mm Hg lisinopril in patients with severe hypertension (mean (95% CI À1.7, 7.4)). Responder rates (msDBPo90 mm Hg sitting diastolic blood pressure (msDBP)X105 mm Hg and/or reduction from baselineX10 mm Hg) were 81.5% and o120 mm Hg). -

"Coaprovel, INN-Irbesartan+Hydrochlorothiazide"
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT CoAprovel 150 mg/12.5 mg tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 150 mg of irbesartan and 12.5 mg of hydrochlorothiazide. Excipient with known effect: Each tablet contains 26.65 mg of lactose (as lactose monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. Peach, biconvex, oval-shaped, with a heart debossed on one side and the number 2775 engraved on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of essential hypertension. This fixed dose combination is indicated in adult patients whose blood pressure is not adequately controlled on irbesartan or hydrochlorothiazide alone (see section 5.1). 4.2 Posology and method of administration Posology CoAprovel can be taken once daily, with or without food. Dose titration with the individual components (i.e. irbesartan and hydrochlorothiazide) may be recommended. When clinically appropriate direct change from monotherapy to the fixed combinations may be considered: . CoAprovel 150 mg/12.5 mg may be administered in patients whose blood pressure is not adequately controlled with hydrochlorothiazide or irbesartan 150 mg alone; . CoAprovel 300 mg/12.5 mg may be administered in patients insufficiently controlled by irbesartan 300 mg or by CoAprovel 150 mg/12.5 mg. CoAprovel 300 mg/25 mg may be administered in patients insufficiently controlled by CoAprovel 300 mg/12.5 mg. Doses higher than 300 mg irbesartan/25 mg hydrochlorothiazide once daily are not recommended. When necessary, CoAprovel may be administered with another antihypertensive medicinal product (see sections 4.3, 4.4, 4.5 and 5.1). -

Summary of Product Characteristics 1. Name Of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules Ramipril/Amlodipine Glenmark, 10 mg/10 mg, hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules: each capsule contains 5 mg ramipril and amlodipine besilate equivalent to 5 mg amlodipine. Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules: each capsule contains 5 mg ramipril and amlodipine besilate equivalent to 10 mg amlodipine and . Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules: each capsule contains 10 mg ramipril and amlodipine besilate equivalent to5 mg amlodipine. Ramipril/Amlodipine Glenmark, 10 mg/10 mg, hard capsules: each capsule contains 10 mg ramipril and amlodipine besilate equivalent to 10 mg amlodipine . For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Hard capsule Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque pink colour, body: opaque white colour. Content of capsules: white or almost white powder. Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque red - brown colour, body: opaque white colour. Content of capsules: white or almost white powder. Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque dark pink colour, body: opaque white colour. Content of capsules: white or almost white powder. -

Medication Risks in Older Patients with Cancer
Medication risks in older patients with cancer 1 Medication risks in older patients (70+) with cancer and their association with therapy-related toxicity Imke Ortland1, Monique Mendel Ott1, Michael Kowar2, Christoph Sippel3, Yon-Dschun Ko3#, Andreas H. Jacobs2#, Ulrich Jaehde1# 1 Institute of Pharmacy, Department of Clinical Pharmacy, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany 2 Department of Geriatrics and Neurology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany 3 Department of Oncology and Hematology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany # equal contribution Corresponding author Ulrich Jaehde Institute of Pharmacy University of Bonn An der Immenburg 4 53121 Bonn, Germany Phone: +49 228-73-5252 Fax: +49-228-73-9757 [email protected] Medication risks in older patients with cancer 2 Abstract Objectives To evaluate medication-related risks in older patients with cancer and their association with severe toxicity during antineoplastic therapy. Methods This is a secondary analysis of two prospective, single-center observational studies which included patients ≥ 70 years with cancer. The patients’ medication was investigated regarding possible risks: polymedication (defined as the use of ≥ 5 drugs), potentially inadequate medication (PIM; defined by the EU(7)-PIM list), and relevant potential drug- drug interactions (rPDDI; analyzed by the ABDA interaction database). The risks were analyzed at two different time points: before and after start of cancer therapy. Severe toxicity during antineoplastic therapy was captured from medical records according to the Common Terminology Criteria for Adverse Events (CTCAE). The association between Grade ≥ 3 toxicity and medication risks was evaluated by univariate regression. -

Summary of Product Characteristics 1. NAME of the MEDICINAL
Summary of product characteristics 1. NAME OF THE MEDICINAL PRODUCT Losartan Potassium 50 mg Film-coated Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 50 mg of losartan potassium, equivalent to 45.8mg of Losartan. Excipient: 52mg of lactose/film-coated tablet. For the full list of excipients see section 6.1 3. PHARMACEUTICAL FORM Film-coated tablet White to off white, round, biconvex, film-coated tablets with breakline on one side and “50” debossing on other side. The break line is only to facilitate breaking for ease of swallowing and not to divide into equal doses 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of essential hypertension in adults and in children and adolescents 6-18 years of age. Treatment of renal disease in adult patients with hypertension and type 2 diabetes mellitus with proteinuria 0.5 g/day as part of an antihypertensive treatment (see sections 4.3, 4.4, 4.5 and 5.1). Treatment of chronic heart failure in adult patients when treatment with Angiotensin converting enzyme (ACE) inhibitors is not considered suitable due to incompatibility, especially cough, or contraindication. Patients with heart failure who have been stabilised with an ACE inhibitor should not be switched to losartan. The patients should have a left ventricular ejection fraction ≤ 40% and should be clinically stable and on an established treatment regimen for chronic heart failure. Reduction in the risk of stroke in adult hypertensive patients with left ventricular hypertrophy documented by ECG (see section 5.1 LIFE study, Race). 4.2 Posology and method of administration Posology Hypertension The usual starting and maintenance dose is 50 mg once daily for most patients. -
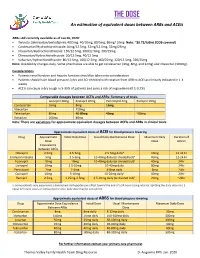
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

COZAAR (Losartan Potassium) Tablets, for Oral Use
HIGHLIGHTS OF PRESCRIBING INFORMATION • Increase dose to 100 mg once daily if further blood pressure These highlights do not include all the information needed to use response is needed. (2.3) COZAAR safely and effectively. See full prescribing information for COZAAR. --------------------- DOSAGE FORMS AND STRENGTHS --------------------- Tablets: 25 mg; 50 mg; and 100 mg. (3) ® COZAAR (losartan potassium) tablets, for oral use ------------------------------- CONTRAINDICATIONS ------------------------------- Initial U.S. Approval: 1995 • Hypersensitivity to any component. (4) • Coadministration with aliskiren in patients with diabetes. (4) WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. ----------------------- WARNINGS AND PRECAUTIONS ----------------------- • Hypotension: Correct volume or salt depletion prior to administration When pregnancy is detected, discontinue COZAAR as soon as of COZAAR. (5.2) possible. Drugs that act directly on the renin-angiotensin system • Monitor renal function and potassium in susceptible patients. (5.3, can cause injury and death to the developing fetus. (5.1) 5.4) --------------------------- RECENT MAJOR CHANGES --------------------------- ------------------------------ ADVERSE REACTIONS ------------------------------ Warnings and Precautions Hyperkalemia (5.4) 10/2018 Most common adverse reactions (incidence ≥2% and greater than placebo) are: dizziness, upper respiratory infection, nasal congestion, ----------------------------INDICATIONS AND USAGE ---------------------------- and back pain. (6.1) COZAAR is an angiotensin II receptor blocker (ARB) indicated for: • Treatment of hypertension, to lower blood pressure in adults and To report SUSPECTED ADVERSE REACTIONS, contact Merck children greater than 6 years old. Lowering blood pressure reduces Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877- the risk of fatal and nonfatal cardiovascular events, primarily strokes 888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

Angiotensin-Converting Enzyme (ACE) Inhibitors
Angiotensin-Converting Enzyme (ACE) Inhibitors Summary Blood pressure reduction is similar for the ACE inhibitors class, with no clinically meaningful differences between agents. Side effects are infrequent with ACE inhibitors, and are usually mild in severity; the most commonly occurring include cough and hypotension. Captopril and lisinopril do not require hepatic conversion to active metabolites and may be preferred in patients with severe hepatic impairment. Captopril differs from other oral ACE inhibitors in its rapid onset and shorter duration of action, which requires it to be given 2-3 times per day; enalaprilat, an injectable ACE inhibitor also has a rapid onset and shorter duration of action. Pharmacology Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) block the conversion of angiotensin I to angiotensin II through competitive inhibition of the angiotensin converting enzyme. Angiotensin is formed via the renin-angiotensin-aldosterone system (RAAS), an enzymatic cascade that leads to the proteolytic cleavage of angiotensin I by ACEs to angiotensin II. RAAS impacts cardiovascular, renal and adrenal functions via the regulation of systemic blood pressure and electrolyte and fluid balance. Reduction in plasma levels of angiotensin II, a potent vasoconstrictor and negative feedback mediator for renin activity, by ACE inhibitors leads to increased plasma renin activity and decreased blood pressure, vasopressin secretion, sympathetic activation and cell growth. Decreases in plasma angiotensin II levels also results in a reduction in aldosterone secretion, with a subsequent decrease in sodium and water retention.[51035][51036][50907][51037][24005] ACE is found in both the plasma and tissue, but the concentration appears to be greater in tissue (primarily vascular endothelial cells, but also present in other organs including the heart).