Part1 Gendiff.Qxp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ambell-5 Amlodipine Besylate Tablets Usp 5 Mg
AMBELL-5 AMLODIPINE BESYLATE TABLETS USP 5 MG 1. NAME OF THE MEDICINAL PRODUCT AMBELL-5 Amlodipine Besylate Tablets USP 5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each uncoated tablet contains: Amlodipine Besilate USP Eq. to Amlodipine………….5 mg Excipients…………………..q.s. For a full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM Uncoated Tablets 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Hypertension - Chronic stable and vasospastic anginal pectoris - Vasospastic (Prinzmetal's) angina 4.2 Posology and method of administration Posology Adults For both hypertension and angina the usual initial dose is 5 mg Amlodipine once daily which may be increased to a maximum dose of 10 mg depending on the individual patient's response. In hypertensive patients, amlodipine has been used in combination with a thiazide diuretic, alpha blocker, beta blocker, or an angiotensin converting enzyme inhibitor. For angina, amlodipine may be used as monotherapy or in combination with other antianginal medicinal products in patients with angina that is refractory to nitrates and/or to adequate doses of beta blockers. No dose adjustment of amlodipine is required upon concomitant administration of thiazide diuretics, beta blockers, and angiotensin-converting enzyme inhibitors. Special populations Elderly Amlodipine used at similar doses in elderly or younger patients is equally well tolerated. Normal dosage regimens are recommended in the elderly, but increase of the dosage should take place with care (see sections 4.4 and 5.2). Patients with hepatic impairment Dosage recommendations have not been established in patients with mild to moderate hepatic impairment, therefore dose selection should be cautious and should start at the lower end of the dosing range (see sections 4.4 and 5.2). -

TRIBAL COUNCIL REPORT COVID-19 TESTING and DISEASE in FIRST NATIONS on RESERVE JULY 26, 2021 *The Reports Covers COVID-19 Testing Since the First Reported Case
TRIBAL COUNCIL REPORT COVID-19 TESTING AND DISEASE IN FIRST NATIONS ON RESERVE JULY 26, 2021 *The reports covers COVID-19 testing since the first reported case. The last TC report provided was on Monday July 19, 2021. DOTC Total Cases 252 Recovered Cases 240 New Cases 1 Active Cases 4 Total Deaths 8 FARHA Total Cases 1833 Recovered Cases 1814 New Cases 1 Active Cases 8 Total Deaths 11 Independent-North Total Cases 991 Recovered Cases 977 New Cases 0 Active Cases 4 Total Deaths 10 This summary report is intended to provide high-level analysis of COVID-19 testing and disease in First Nations on reserve by Tribal Council Region since first case until date noted above. JULY 26, 2021 Independent- South Total Cases 425 Recovered Cases 348 New Cases 36 Active Cases 74 Total Deaths 3 IRTC Total Cases 651 Recovered Cases 601 New Cases 11 Active Cases 38 Total Deaths 12 KTC Total Cases 1306 Recovered Cases 1281 New Cases 1 Active Cases 15 Total Deaths 10 This summary report is intended to provide high-level analysis of COVID-19 testing and disease in First Nations on reserve by Tribal Council Region since first case until date noted above. JULY 26, 2021 SERDC Total Cases 737 Recovered Cases 697 New Cases 14 Active Cases 31 Total Deaths 9 SCTC Total Cases 1989 Recovered Cases 1940 New Cases 11 Active Cases 31 Total Deaths 18 WRTC Total Cases 377 Recovered Cases 348 New Cases 2 Active Cases 25 Total Deaths 4 This summary report is intended to provide high-level analysis of COVID-19 testing and disease in First Nations on reserve by Tribal Council Region since first case until date noted above. -

A Home Away from Home at Grand Beach, Manitoba
Prairie Perspectives 135 A home away from home at Grand Beach, Manitoba John Selwood, University of Winnipeg Matthew Tonts, University of Western Australia Abstract: From its inception, Grand Beach, Manitoba, quickly became one of Western Canada’s foremost recreational, cottage resort localities and it has regained that position despite many years of neglect and abuse. Dominion government surveyors were the first to recognize the recreational possibilities of the beach, but this potential was not realized until the Canadian Northern Railway extended a line up the eastern side of Lake Winnipeg. Under a leasing arrangement with the provincial government, the railway company developed Grand Beach peninsula into an extremely popular lakeside resort and camping area, which during its heyday, was visited by countless thousands of excursionists and longer-term vacationers. This paper focuses on the development of the ‘Campsite’, an area initially created for temporary summer campers, but which was soon converted to leasehold lots available for long-term cottage development. Although the area is now part of Grand Beach Provincial Park, the cottages built in the old campground still remain. However, they are now being progressively upgraded, converted into more permanent fixtures, and many adapted for year-round use. Surveys of cottagers identify some interesting ownership patterns and reveal the strength of attachment that many of the predominantly urban residents have for their cottages or second homes, as they are commonly referred to in the academic literature. Introduction Although it is best known as a popular day resort area, Grand Beach is also one of Manitoba’s more important areas of cottage development, having been developed nearly a century ago during the First World War. -

Dyslipidemia in Newfoundland: Findings from Canadian Primary Care Sentinel Surveillance Network in Newfoundland and Labrador
Dyslipidemia in Newfoundland: Findings from Canadian Primary Care Sentinel Surveillance Network in Newfoundland and Labrador By Justin D. Oake A thesis submitted to the School of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science in Medicine Clinical Epidemiology Program, Faculty of Medicine, Memorial University of Newfoundland St. John’s, NL May 2019 Abstract Newfoundland and Labrador (NL) has a higher level of cardiovascular disease (CVD) mortality than any other Canadian province. One factor which may explain this trend is the lipid profile pattern in this province. Given the limited lipid profile data which has been reported from NL, we organized three studies in this thesis to describe the lipid profile of Newfoundlanders. The first study was a secondary analysis of Canadian Primary Care Sentinel Surveillance Network (CPCSSN) data to document single and mixed dyslipidemia in NL. The second study compared lipid profiles and the prevalence of dyslipidemia between NL CPCSSN data and the Canadian Health Measures Survey (CHMS). The third study used electronic medical record (EMR) data in assessing the validity of ICD codes for identifying patients with dyslipidemia. This was a secondary analysis of EMR data in NL. Most recent lipid profile scores, co-morbidities, and demographic information were extracted from the CPCSSN database. We demonstrated that single and mixed dyslipidemia are quite prevalent in the NL population. Unhealthy levels of HDL were also more prevalent in NL men, compared to the Canadian sample. Of importance, the use of the ICD coding, either alone or in combination with laboratory data or lipid-lowering medication records, was an inaccurate indicator in identifying dyslipidemia. -

Chapter 4 – Project Setting
Chapter 4 – Project Setting MINAGO PROJECT i Environmental Impact Statement TABLE OF CONTENTS 4. PROJECT SETTING 4-1 4.1 Project Location 4-1 4.2 Physical Environment 4-2 4.3 Ecological Characterization 4-3 4.4 Social and Cultural Environment 4-5 LIST OF FIGURES Figure 4.1-1 Property Location Map ......................................................................................................... 4-1 Figure 4.4-1 Communities of Interest Surveyed ....................................................................................... 4-6 MINAGO PROJECT ii Environmental Impact Statement VICTORY NICKEL INC. 4. PROJECT SETTING 4.1 Project Location The Minago Nickel Property (Property) is located 485 km north-northwest of Winnipeg, Manitoba, Canada and 225 km south of Thompson, Manitoba on NTS map sheet 63J/3. The property is approximately 100 km north of Grand Rapids off Provincial Highway 6 in Manitoba. Provincial Highway 6 is a paved two-lane highway that serves as a major transportation route to northern Manitoba. The site location is shown in Figure 4.1-1. Source: Wardrop, 2006 Figure 4.1-1 Property Location Map MINAGO PROJECT 4-1 Environmental Impact Statement VICTORY NICKEL INC. 4.2 Physical Environment The Minago Project is located within the Nelson River sub-basin, which drains northeast into the southern end of the Hudson Bay. The Minago River and Hargrave River catchments, surrounding the Minago Project Site to the north, occur within the Nelson River sub-basin. The William River and Oakley Creek catchments at or surrounding the Minago Project Site to the south, occur within the Lake Winnipeg sub-basin, which flows northward into the Nelson River sub-basin. The topography in these watersheds varies between elevation 210 and 300 m.a.s.l. -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

Summary of Product Characteristics
Proposed var 24 psusa cilazapril SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [Fosinopril sodium 10 mg, tablets] [Fosinopril sodium 20 mg, tablets] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 or 20 mg fosinopril sodium. Excipient with known effect: Each tablet fosinopril sodium 10 mg contains 87 mg of lactose, anhydrous. Each tablet fosinopril sodium 20 mg contains 174 mg of lactose, anhydrous. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. The 10 mg tablets are white and shaped like a capsule with indents. On one side they are engraved with the letters “APO” and on the other side with “FOS-10”. The 20 mg tablets are white and their shape is oval. On one side they are engraved with the letters “APO” and on the other side with “FOS-20”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Treatment of hypertension. - Treatment of symptomatic heart failure. 4.2 Posology and method of administration Posology Fosinopril sodium should be administered orally in a single daily dose. As with all other medicinal products taken once daily, it should be taken at approximately the same time each day. The absorption of fosinopril sodium is not affected by food. The dose should be individualised according to patient profile and blood pressure response (see section 4.4). Hypertension: Fosinopril sodium may be used as a monotherapy or in combination with other classes of antihypertensive medicinal products (see section 4.3, 4.4, 4.5 and 5.1). Hypertensive patients not being treated with diuretics: Starting dose The initial recommended dose is 10 mg once a day. -

MEETING CODES EASTERVILLE Town Hall Bsmt
MINNEDOSA Tuesday 8:00 PM C MEETING CODES EASTERVILLE Town Hall bsmt. 103 A. South Main St Last Tues. Birthday 8:00 PM Sp O Chemawawin School Thursday 6:00 PM D C C – Closed for alcoholics only; O – open to all; D – Discussion; BB – Big Book; T – Tradition; S – Step; MORDEN ELPHINSTONE Fresh Air Group (WA) Meetings suspended until further notice B – Beginners; Sp – Speaker; WA – Wheelchair Accessible KeeSee Group Tuesday 7:00 PM D O St. Thomas Anglican Church 131-8th Street Health Center NEEPAWA GROUP NAME & MEETING LOCATION DAYS TIME TYPE ERIKSDALE Hospital boardroom (basement) Thursday 7:30 PM D C 14 Railway Avenue New Horizon Bldg. Monday 7:00 PM O ALTONA Altona Group Monday 8:00 PM C FALCON LAKE NELSON HOUSE Basement of United Church Whiteshell Group Meetings suspended until further notice Sharing Feather Group Medicine Lodge Wed. & Thurs. 8:00 PM D C All People’s Church (side door) AMARANTH NIVERVILLE Sandy Bay Tuesday 7:00 PM C FISHER RIVER Niverville Big Book Thursday 7:00 PM BB C Fisher River Group Tuesday 6:00 PM D C Niverville Community Fellowship ARBORG Corner of 2nd Ave. S & 2nd St. S. Arborg Group Thursday 8:30 PM D C FLIN FLON NORWAY HOUSE Support Centre, 327 Main St. Eastside Group 201 Bracken St. Mon, Wed, Fri 7:30 PM S C Lighthouse Group Tues & Thur 8:00 PM BARROWS Wednesday 8:00 PM C GIMLI OAKBANK Barrows Group Gimli Group Meetings Suspended Until further notice Oakbank Group Scent Free Tuesday 7:30 PM BB C United Church Basement 582 Balsam Cres. -

Effects of Generic Substitution on Refill Adherence to Statin Therapy: a Nationwide Population-Based Study Henrik Trusell1 and Karolina Andersson Sundell1,2*
Trusell and Andersson Sundell BMC Health Services Research 2014, 14:626 http://www.biomedcentral.com/1472-6963/14/626 RESEARCH ARTICLE Open Access Effects of generic substitution on refill adherence to statin therapy: a nationwide population-based study Henrik Trusell1 and Karolina Andersson Sundell1,2* Abstract Background: Several countries have introduced generic substitution, but few studies have assessed its effect on refill adherence. This study aimed to analyse whether generic substitution influences refill adherence to statin treatment. Methods: Between 1 July 2006 and 30 June 2007, new users of simvastatin (n = 108,806) and atorvastatin (n = 7,464) were identified in the Swedish Prescribed Drug Register . The present study included atorvastatin users as an unexposed control group because atorvastatin was patent-protected and thus not substitutable. We assessed refill adherence using continuous measure of medication acquisition (CMA). To control for potential confounders, we used analysis of covariance (ANCOVA). Differences in CMA associated with generic substitution and generic substitution at first-time statin purchase were analysed. Results: Nine of ten simvastatin users were exposed to generic substitution during the study period, and their adherence rate was higher than that of patients without substitution [84.6% (95% CI 83.5-85.6) versus 59.9% (95% CI 58.4-61.4), p < 0.001]. CMA was higher with increasing age (60–69 years 16.7%, p < 0.0001 and 70–79 years 17.8%, p < 0.0001, compared to 18–39 years) and secondary prevention (12.8%, p < 0.0001). CMA was lower among patients who were exposed to generic substitution upon initial purchase, compared to those who were exposed to a generic substitution subsequently [80.4% (95% CI 79.4-90.9) versus 89.8% (88.7-90.9), p < 0.001]. -

Manitoba Regional Health Authority (RHA) DISTRICTS MCHP Area Definitions for the Period 2002 to 2012
Manitoba Regional Health Authority (RHA) DISTRICTS MCHP Area Definitions for the period 2002 to 2012 The following list identifies the RHAs and RHA Districts in Manitoba between the period 2002 and 2012. The 11 RHAs are listed using major headings with numbers and include the MCHP - Manitoba Health codes that identify them. RHA Districts are listed under the RHA heading and include the Municipal codes that identify them. Changes / modifications to these definitions and the use of postal codes in definitions are noted where relevant. 1. CENTRAL (A - 40) Note: In the fall of 2002, Central changed their districts, going from 8 to 9 districts. The changes are noted below, beside the appropriate district area. Seven Regions (A1S) (* 2002 changed code from A8 to A1S *) '063' - Lakeview RM '166' - Westbourne RM '167' - Gladstone Town '206' - Alonsa RM 'A18' - Sandy Bay FN Cartier/SFX (A1C) (* 2002 changed name from MacDonald/Cartier, and code from A4 to A1C *) '021' - Cartier RM '321' - Headingley RM '127' - St. Francois Xavier RM Portage (A1P) (* 2002 changed code from A7 to A1P *) '090' - Macgregor Village '089' - North Norfolk RM (* 2002 added area from Seven Regions district *) '098' - Portage La Prairie RM '099' - Portage La Prairie City 'A33' - Dakota Tipi FN 'A05' - Dakota Plains FN 'A04' - Long Plain FN Carman (A2C) (* 2002 changed code from A2 to A2C *) '034' - Carman Town '033' - Dufferin RM '053' - Grey RM '112' - Roland RM '195' - St. Claude Village '158' - Thompson RM 1 Manitoba Regional Health Authority (RHA) DISTRICTS MCHP Area -

Marie Louise De Bruin Drug Induced Arrhythmias
Marie Louise De Bruin drug induced arrhythmias Quantifying the problem Cover design: Tom Frantzen CIP-gegevens Koninklijke Bibliotheek, Den Haag De Bruin, Marie Louise Drug-induced arrhythmias, quantifying the problem / Marie Louise De Bruin Thesis Utrecht - with ref.- with summary in Dutch ISBN: 90-808203-3-4 © Marie Louise De Bruin drug-induced arrhythmias, quantifying the problem Geneesmiddel-geïnduceerde hartritmestoornissen, kwantificering van het probleem (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit van Utrecht op gezag van de Rector Magnificus Prof. dr W.H. Gispen, ingevolge het besluit van het College voor Promoties in het openbaar te verdedigen op woensdag 1 december 2004 des namiddags om 14.30 uur door Marie Louise De Bruin Geboren op 1 april 1974 te Haarlem promotores Prof. dr H.G.M. Leufkens Utrecht Institute for Pharmaceutical Sciences (UIPS), Department of Pharmaco- epidemiology and Pharmacotherapy, Utrecht University, Utrecht, the Netherlands Prof. dr A.W. Hoes Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, the Netherlands The work in this thesis was performed at the Department of Pharmacoepidemiology and Pharmacotherapy of the Utrecht Institute for Pharmaceutical Sciences (Utrecht) and the Julius Center for Health Sciences and Primary Care (Utrecht), in collabo- ration with the PHARMO Institute (Utrecht), the Netherlands Pharmacovigilance Centre Lareb (‘s-Hertogenbosch), the WHO-Uppsala Monitoring Centre (Uppsala, Sweden) and the Academic Medical Center (Amsterdam). The research presented in this thesis was funded by the Utrecht Institute for Pharmaceutical Sciences, and an unrestricted grant from the Dutch Medicines Evaluation Board. The study presented in chapter 3.1 was funded by an unrestricted grant from Janssen Pharmaceutica NV, Beerse, Belgium. -
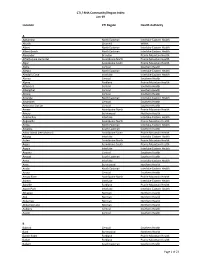
CTI / RHA Community/Region Index Jan-19
CTI / RHA Community/Region Index Jan-19 Location CTI Region Health Authority A Aghaming North Eastman Interlake-Eastern Health Akudik Churchill WRHA Albert North Eastman Interlake-Eastern Health Albert Beach North Eastman Interlake-Eastern Health Alexander Brandon Prairie Mountain Health Alfretta (see Hamiota) Assiniboine North Prairie Mountain Health Algar Assiniboine South Prairie Mountain Health Alpha Central Southern Health Allegra North Eastman Interlake-Eastern Health Almdal's Cove Interlake Interlake-Eastern Health Alonsa Central Southern Health Alpine Parkland Prairie Mountain Health Altamont Central Southern Health Albergthal Central Southern Health Altona Central Southern Health Amanda North Eastman Interlake-Eastern Health Amaranth Central Southern Health Ambroise Station Central Southern Health Ameer Assiniboine North Prairie Mountain Health Amery Burntwood Northern Health Anama Bay Interlake Interlake-Eastern Health Angusville Assiniboine North Prairie Mountain Health Anola North Eastman Interlake-Eastern Health Arbakka South Eastman Southern Health Arbor Island (see Morton) Assiniboine South Prairie Mountain Health Arborg Interlake Interlake-Eastern Health Arden Assiniboine North Prairie Mountain Health Argue Assiniboine South Prairie Mountain Health Argyle Interlake Interlake-Eastern Health Arizona Central Southern Health Amaud South Eastman Southern Health Ames Interlake Interlake-Eastern Health Amot Burntwood Northern Health Anola North Eastman Interlake-Eastern Health Arona Central Southern Health Arrow River Assiniboine