Marie Louise De Bruin Drug Induced Arrhythmias
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ambell-5 Amlodipine Besylate Tablets Usp 5 Mg
AMBELL-5 AMLODIPINE BESYLATE TABLETS USP 5 MG 1. NAME OF THE MEDICINAL PRODUCT AMBELL-5 Amlodipine Besylate Tablets USP 5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each uncoated tablet contains: Amlodipine Besilate USP Eq. to Amlodipine………….5 mg Excipients…………………..q.s. For a full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM Uncoated Tablets 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Hypertension - Chronic stable and vasospastic anginal pectoris - Vasospastic (Prinzmetal's) angina 4.2 Posology and method of administration Posology Adults For both hypertension and angina the usual initial dose is 5 mg Amlodipine once daily which may be increased to a maximum dose of 10 mg depending on the individual patient's response. In hypertensive patients, amlodipine has been used in combination with a thiazide diuretic, alpha blocker, beta blocker, or an angiotensin converting enzyme inhibitor. For angina, amlodipine may be used as monotherapy or in combination with other antianginal medicinal products in patients with angina that is refractory to nitrates and/or to adequate doses of beta blockers. No dose adjustment of amlodipine is required upon concomitant administration of thiazide diuretics, beta blockers, and angiotensin-converting enzyme inhibitors. Special populations Elderly Amlodipine used at similar doses in elderly or younger patients is equally well tolerated. Normal dosage regimens are recommended in the elderly, but increase of the dosage should take place with care (see sections 4.4 and 5.2). Patients with hepatic impairment Dosage recommendations have not been established in patients with mild to moderate hepatic impairment, therefore dose selection should be cautious and should start at the lower end of the dosing range (see sections 4.4 and 5.2). -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -
Guidance for the Format and Content of the Protocol of Non-Interventional
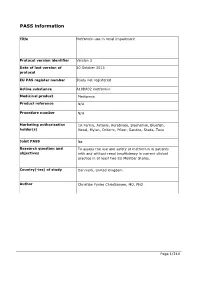
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

Atopic Children and Use of Prescribed Medication: a Comprehensive Study in General Practice
RESEARCH ARTICLE Atopic children and use of prescribed medication: A comprehensive study in general practice David H. J. Pols1*, Mark M. J. Nielen2, Arthur M. Bohnen1, Joke C. Korevaar2, Patrick J. E. Bindels1 1 Department of General Practice, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands, 2 NIVEL, Netherlands Institute for Health Services Research, Utrecht, The Netherlands a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 Purpose A comprehensive and representative nationwide general practice database was explored to study associations between atopic disorders and prescribed medication in children. OPEN ACCESS Citation: Pols DHJ, Nielen MMJ, Bohnen AM, Korevaar JC, Bindels PJE (2017) Atopic children Method and use of prescribed medication: A All children aged 0±18 years listed in the NIVEL Primary Care Database in 2014 were comprehensive study in general practice. PLoS ONE 12(8): e0182664. https://doi.org/10.1371/ selected. Atopic children with atopic eczema, asthma and allergic rhinitis (AR) were journal.pone.0182664 matched with controls (not diagnosed with any of these disorders) within the same general Editor: Anthony Peter Sampson, University of practice on age and gender. Logistic regression analyses were performed to study the differ- Southampton School of Medicine, UNITED ences in prescribed medication between both groups by calculating odds ratios (OR); 93 dif- KINGDOM ferent medication groups were studied. Received: March 24, 2017 Accepted: July 13, 2017 Results Published: August 24, 2017 A total of 45,964 children with at least one atopic disorder were identified and matched with Copyright: © 2017 Pols et al. This is an open controls. Disorder-specific prescriptions seem to reflect evidence-based medicine guide- access article distributed under the terms of the lines for atopic eczema, asthma and AR. -

Evaluating Onco-Geriatric Scores and Medication Risks to Improve Cancer Care for Older Patients
Evaluating onco-geriatric scores and medication risks to improve cancer care for older patients Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von IMKE ORTLAND aus Quakenbrück Bonn 2019 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn. Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn elektronisch publiziert. https://nbn-resolving.org/urn:nbn:de:hbz:5-58042 Erstgutachter: Prof. Dr. Ulrich Jaehde Zweitgutachter: Prof. Dr. Andreas Jacobs Tag der Promotion: 28. Februar 2020 Erscheinungsjahr: 2020 Danksagung Auf dem Weg zur Promotion haben mich viele Menschen begleitet und in ganz unterschiedlicher Weise unterstützt. All diesen Menschen möchte ich an dieser Stelle ganz herzlich danken. Mein aufrichtiger Dank gilt meinem Doktorvater Prof. Dr. Ulrich Jaehde für das in mich gesetzte Vertrauen, sowie für die Überlassung dieses spannenden Dissertationsthemas. Die uneingeschränkte Unterstützung, wertvollen Diskussionen und die mitreißende Begeisterung für die Wissenschaft haben mich während aller Phasen der Dissertation stets motiviert, unterstützt und sehr viel Wertvolles gelehrt. Prof. Dr. Andreas Jacobs danke ich herzlich für die Initiierung dieses interessanten Projekts, für die stetige Begeisterung und Unterstützung, sowie für das mir entgegengebrachte Vertrauen. Die ausgezeichnete Zusammenarbeit mit dem Johanniter Krankenhaus Bonn hat ganz maßgeblich zum Gelingen dieser Arbeit beigetragen. Auch danke ich Prof. Dr. Andreas Jacobs herzlich für die Bereitschaft, das Koreferat dieser Arbeit zu übernehmen. Ebenfalls danke ich herzlich Prof. Dr. Yon-Dschun Ko für seine fortwährende Motivation und seinen Einsatz, sowie für das mir geschenkte Vertrauen, dieses Projekt am Johanniter Krankenhaus zu realisieren. Ebenfalls danke ich Prof. -

Quality Issues in Caring for Older People
Doctoral Thesis - Tesis Doctoral Quality issues in caring for older people: • Appropriateness of transition from long-term care facilities to acute hospital care • Potentially inappropriate medication: development of a European list Anna Renom Guiteras Prof. Gabriele Meyer Prof. Ramón Miralles Basseda Martin Luther University Halle-Wittenberg Universitat Autònoma de Barcelona Halle (Saale) & Barcelona, Catalonia University of Witten/Herdecke Spain Witten Germany Programa de doctorat en Medicina Departament de Medicina, Facultat de Medicina Universitat Autònoma de Barcelona Barcelona, 2015 13 Contents 15 1. Introduction • Research context • Background of the research topics • Pesetaio of the ailes 23 2. Summary and discussion of the results 31 3. Conclusions 37 4. References 47 5. Articles • Article 1: Renom-Guiteras A, Uhrenfeldt L, Meyer G, Mann E. Assessment tools for determining appropriateness of admission to acute care of persons transferred from long-term care facilities: a systematic review. BMC Geriatr. 2014;14:80 • Article 2: Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71(7):861-75 77 6. Annexes • Annex 1.1 (article 1) - Additional file 1: Studies dealing with assessment tools for determining appropriateness of hospital admissions among residents of LTC facilities. • Annex 1.2 (article 1) - Additional file 2: Characteristics of the assessment tools for determining appropriateness of hospital admissions among residents of LTC facilities. • Annex 2.1 (article 2) - Appendix 1: Complete EU(7)-PIM list • Annex 2.2 (article 2) - Appendix 2: Questionable Potentially Inappropriate Medications (Questionable PIM): results of the Delphi survey. -

Medication Risks in Older Patients with Cancer
Medication risks in older patients with cancer 1 Medication risks in older patients (70+) with cancer and their association with therapy-related toxicity Imke Ortland1, Monique Mendel Ott1, Michael Kowar2, Christoph Sippel3, Yon-Dschun Ko3#, Andreas H. Jacobs2#, Ulrich Jaehde1# 1 Institute of Pharmacy, Department of Clinical Pharmacy, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany 2 Department of Geriatrics and Neurology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany 3 Department of Oncology and Hematology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany # equal contribution Corresponding author Ulrich Jaehde Institute of Pharmacy University of Bonn An der Immenburg 4 53121 Bonn, Germany Phone: +49 228-73-5252 Fax: +49-228-73-9757 [email protected] Medication risks in older patients with cancer 2 Abstract Objectives To evaluate medication-related risks in older patients with cancer and their association with severe toxicity during antineoplastic therapy. Methods This is a secondary analysis of two prospective, single-center observational studies which included patients ≥ 70 years with cancer. The patients’ medication was investigated regarding possible risks: polymedication (defined as the use of ≥ 5 drugs), potentially inadequate medication (PIM; defined by the EU(7)-PIM list), and relevant potential drug- drug interactions (rPDDI; analyzed by the ABDA interaction database). The risks were analyzed at two different time points: before and after start of cancer therapy. Severe toxicity during antineoplastic therapy was captured from medical records according to the Common Terminology Criteria for Adverse Events (CTCAE). The association between Grade ≥ 3 toxicity and medication risks was evaluated by univariate regression. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Part1 Gendiff.Qxp
Sex Differences in Health Status, Health Care Use, and Quality of Care: A Population-Based Analysis for Manitoba’s Regional Health Authorities November 2005 Manitoba Centre for Health Policy Department of Community Health Sciences Faculty of Medicine, University of Manitoba Randy Fransoo, MSc Patricia Martens, PhD The Need to KnowTeam (funded through CIHR) Elaine Burland, MSc Heather Prior, MSc Charles Burchill, MSc Dan Chateau, PhD Randy Walld, BSc, BComm (Hons) This report is produced and published by the Manitoba Centre for Health Policy (MCHP). It is also available in PDF format on our website at http://www.umanitoba.ca/centres/mchp/reports.htm Information concerning this report or any other report produced by MCHP can be obtained by contacting: Manitoba Centre for Health Policy Dept. of Community Health Sciences Faculty of Medicine, University of Manitoba 4th Floor, Room 408 727 McDermot Avenue Winnipeg, Manitoba, Canada R3E 3P5 Email: [email protected] Order line: (204) 789 3805 Reception: (204) 789 3819 Fax: (204) 789 3910 How to cite this report: Fransoo R, Martens P, The Need To Know Team (funded through CIHR), Burland E, Prior H, Burchill C, Chateau D, Walld R. Sex Differences in Health Status, Health Care Use and Quality of Care: A Population-Based Analysis for Manitoba’s Regional Health Authorities. Winnipeg, Manitoba Centre for Health Policy, November 2005. Legal Deposit: Manitoba Legislative Library National Library of Canada ISBN 1-896489-20-6 ©Manitoba Health This report may be reproduced, in whole or in part, provided the source is cited. 1st Printing 10/27/2005 THE MANITOBA CENTRE FOR HEALTH POLICY The Manitoba Centre for Health Policy (MCHP) is located within the Department of Community Health Sciences, Faculty of Medicine, University of Manitoba. -

Study Protocol and Statistical Analysis Plan
Multicenter double-blind placebo-controlled randomized parallel-group clinical study of efficacy and safety of MMH-MAP in the treatment of mild cognitive impairment in subjects in early rehabilitation period of ischemic stroke Phase III Sponsor OOO «NPF «MATERIA MEDICA HOLDING» Protocol number MMH-MAP-001 Version date: August 29, 2018 ClinicalTrials.gov Id: NCT03815292 Protocol No. MMH-MAP-001 29-Aug-2018 Protocol Summary This document represents the protocol summary for the study on human subjects. The study will be carried out in accordance with ICH GCP, National Standard of the Russian Federation GOST 52379-2005 "Good Clinical Practice", World Medical Association Declaration of Helsinki, relevant requirements of the regulatory authorities as well as the study procedures. Title of Study Multicenter double-blind placebo-controlled randomized parallel-group clinical study of efficacy and safety of MMH-MAP in the treatment of mild cognitive impairment in subjects in early rehabilitation period of ischemic stroke. Phase: III Sponsor: Company «MATERIA MEDICA HOLDING», Moscow, Russia Protocol No. MMH-MAP-001 Objective of the study To evaluate efficacy of MMH-MAP in the treatment of mild cognitive impairment in subjects in early rehabilitation period of ischemic stroke. To evaluate the safety of MMH-MAP in the treatment of mild cognitive impairment in subjects in early rehabilitation period of ischemic stroke. Endpoints Primary endpoint 1. Proportion of subjects with improved cognitive functions (MoCA ≥ +1) after 24-week therapy compared to baseline. Secondary endpoints 1. Change in MoCA scores after 24 weeks of treatment. 2. Percentage of patients with improved performance in activities of daily living (total Barthel Index score + 5 or more) after 24 weeks of treatment. -

Summary of Product Characteristics
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Nitro "Pohl" Infus voor perfusorpompsystemen 1 mg/ml, oplossing voor infusie 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution for infusion contains 1 mg of glyceryl trinitrate (trinitrine), 1 ampoule of 5 ml contains 5 mg trinitrine, 1 ampoule of 10 ml contains 10 mg trinitrine, 1 ampoule of 25 ml contains 25 mg trinitrine, 1 vial of 50 ml contains 50 mg trinitrine. Excipient: glucose monohydrate (0.05 g/ml) For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for infusion, clear and colourless solution 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Surgery/anaesthesia: Prevention of myocardial ischemia during coronary interventions. Cardiology: 1. Left ventricular heart failure, in particular during the acute phase of the myocardial infarction. 2. Unstable angina pectoris 4.2 Posology and method of administration General: It is recommended to start with a low dose of 5 µg/min and increase it gradually every 5 minutes. The dosage must be determined by the patient’s individual requirement and depending on the required response and possible adverse effects, including increased heart rate and hypotension. The dosages determined for individual patients may differ by a factor of 10. In most cases the dosage ranges from 5 - 200 µg/min. Careful clinical monitoring and measurement of blood pressure are necessary for correct adjustment of the infusion rate. 1 4.3 Contraindications Glyceryl trinitrate must not be used in patients with: - hypersensitivity to glyceryl trinitrate and/or nitro compounds in general or for one of the other (inactive) ingredients; - increased intracranial pressure (head injury or cerebral haemorrhage); - insufficient cerebral perfusion; - constrictive pericarditis or pericardial tamponade; - hypotension, with or without cardiogenic shock (systolic blood pressure below 90 mmHg); - uncorrected hypovolemia; - toxic pulmonary oedema. -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open: first published as 10.1136/bmjopen-2020-039747 on 22 October 2020. Downloaded from BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] http://bmjopen.bmj.com/ on September 26, 2021 by guest. Protected copyright. BMJ Open BMJ Open: first published as 10.1136/bmjopen-2020-039747 on 22 October 2020. Downloaded from Development and internal validation of prognostic models to predict negative health outcomes in older patients with multimorbidity and polypharmacy in general practice ForJournal: peerBMJ Open review only Manuscript ID bmjopen-2020-039747 Article Type: Original research Date Submitted by the 24-Apr-2020