Bayer Pharma AG, Leverkusen, Germany
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ambell-5 Amlodipine Besylate Tablets Usp 5 Mg
AMBELL-5 AMLODIPINE BESYLATE TABLETS USP 5 MG 1. NAME OF THE MEDICINAL PRODUCT AMBELL-5 Amlodipine Besylate Tablets USP 5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each uncoated tablet contains: Amlodipine Besilate USP Eq. to Amlodipine………….5 mg Excipients…………………..q.s. For a full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM Uncoated Tablets 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Hypertension - Chronic stable and vasospastic anginal pectoris - Vasospastic (Prinzmetal's) angina 4.2 Posology and method of administration Posology Adults For both hypertension and angina the usual initial dose is 5 mg Amlodipine once daily which may be increased to a maximum dose of 10 mg depending on the individual patient's response. In hypertensive patients, amlodipine has been used in combination with a thiazide diuretic, alpha blocker, beta blocker, or an angiotensin converting enzyme inhibitor. For angina, amlodipine may be used as monotherapy or in combination with other antianginal medicinal products in patients with angina that is refractory to nitrates and/or to adequate doses of beta blockers. No dose adjustment of amlodipine is required upon concomitant administration of thiazide diuretics, beta blockers, and angiotensin-converting enzyme inhibitors. Special populations Elderly Amlodipine used at similar doses in elderly or younger patients is equally well tolerated. Normal dosage regimens are recommended in the elderly, but increase of the dosage should take place with care (see sections 4.4 and 5.2). Patients with hepatic impairment Dosage recommendations have not been established in patients with mild to moderate hepatic impairment, therefore dose selection should be cautious and should start at the lower end of the dosing range (see sections 4.4 and 5.2). -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

Marie Louise De Bruin Drug Induced Arrhythmias
Marie Louise De Bruin drug induced arrhythmias Quantifying the problem Cover design: Tom Frantzen CIP-gegevens Koninklijke Bibliotheek, Den Haag De Bruin, Marie Louise Drug-induced arrhythmias, quantifying the problem / Marie Louise De Bruin Thesis Utrecht - with ref.- with summary in Dutch ISBN: 90-808203-3-4 © Marie Louise De Bruin drug-induced arrhythmias, quantifying the problem Geneesmiddel-geïnduceerde hartritmestoornissen, kwantificering van het probleem (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit van Utrecht op gezag van de Rector Magnificus Prof. dr W.H. Gispen, ingevolge het besluit van het College voor Promoties in het openbaar te verdedigen op woensdag 1 december 2004 des namiddags om 14.30 uur door Marie Louise De Bruin Geboren op 1 april 1974 te Haarlem promotores Prof. dr H.G.M. Leufkens Utrecht Institute for Pharmaceutical Sciences (UIPS), Department of Pharmaco- epidemiology and Pharmacotherapy, Utrecht University, Utrecht, the Netherlands Prof. dr A.W. Hoes Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, the Netherlands The work in this thesis was performed at the Department of Pharmacoepidemiology and Pharmacotherapy of the Utrecht Institute for Pharmaceutical Sciences (Utrecht) and the Julius Center for Health Sciences and Primary Care (Utrecht), in collabo- ration with the PHARMO Institute (Utrecht), the Netherlands Pharmacovigilance Centre Lareb (‘s-Hertogenbosch), the WHO-Uppsala Monitoring Centre (Uppsala, Sweden) and the Academic Medical Center (Amsterdam). The research presented in this thesis was funded by the Utrecht Institute for Pharmaceutical Sciences, and an unrestricted grant from the Dutch Medicines Evaluation Board. The study presented in chapter 3.1 was funded by an unrestricted grant from Janssen Pharmaceutica NV, Beerse, Belgium. -
Guidance for the Format and Content of the Protocol of Non-Interventional
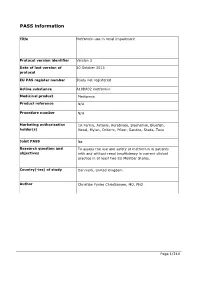
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

Evaluating Onco-Geriatric Scores and Medication Risks to Improve Cancer Care for Older Patients
Evaluating onco-geriatric scores and medication risks to improve cancer care for older patients Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von IMKE ORTLAND aus Quakenbrück Bonn 2019 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn. Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn elektronisch publiziert. https://nbn-resolving.org/urn:nbn:de:hbz:5-58042 Erstgutachter: Prof. Dr. Ulrich Jaehde Zweitgutachter: Prof. Dr. Andreas Jacobs Tag der Promotion: 28. Februar 2020 Erscheinungsjahr: 2020 Danksagung Auf dem Weg zur Promotion haben mich viele Menschen begleitet und in ganz unterschiedlicher Weise unterstützt. All diesen Menschen möchte ich an dieser Stelle ganz herzlich danken. Mein aufrichtiger Dank gilt meinem Doktorvater Prof. Dr. Ulrich Jaehde für das in mich gesetzte Vertrauen, sowie für die Überlassung dieses spannenden Dissertationsthemas. Die uneingeschränkte Unterstützung, wertvollen Diskussionen und die mitreißende Begeisterung für die Wissenschaft haben mich während aller Phasen der Dissertation stets motiviert, unterstützt und sehr viel Wertvolles gelehrt. Prof. Dr. Andreas Jacobs danke ich herzlich für die Initiierung dieses interessanten Projekts, für die stetige Begeisterung und Unterstützung, sowie für das mir entgegengebrachte Vertrauen. Die ausgezeichnete Zusammenarbeit mit dem Johanniter Krankenhaus Bonn hat ganz maßgeblich zum Gelingen dieser Arbeit beigetragen. Auch danke ich Prof. Dr. Andreas Jacobs herzlich für die Bereitschaft, das Koreferat dieser Arbeit zu übernehmen. Ebenfalls danke ich herzlich Prof. Dr. Yon-Dschun Ko für seine fortwährende Motivation und seinen Einsatz, sowie für das mir geschenkte Vertrauen, dieses Projekt am Johanniter Krankenhaus zu realisieren. Ebenfalls danke ich Prof. -

Medication Risks in Older Patients with Cancer
Medication risks in older patients with cancer 1 Medication risks in older patients (70+) with cancer and their association with therapy-related toxicity Imke Ortland1, Monique Mendel Ott1, Michael Kowar2, Christoph Sippel3, Yon-Dschun Ko3#, Andreas H. Jacobs2#, Ulrich Jaehde1# 1 Institute of Pharmacy, Department of Clinical Pharmacy, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany 2 Department of Geriatrics and Neurology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany 3 Department of Oncology and Hematology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany # equal contribution Corresponding author Ulrich Jaehde Institute of Pharmacy University of Bonn An der Immenburg 4 53121 Bonn, Germany Phone: +49 228-73-5252 Fax: +49-228-73-9757 [email protected] Medication risks in older patients with cancer 2 Abstract Objectives To evaluate medication-related risks in older patients with cancer and their association with severe toxicity during antineoplastic therapy. Methods This is a secondary analysis of two prospective, single-center observational studies which included patients ≥ 70 years with cancer. The patients’ medication was investigated regarding possible risks: polymedication (defined as the use of ≥ 5 drugs), potentially inadequate medication (PIM; defined by the EU(7)-PIM list), and relevant potential drug- drug interactions (rPDDI; analyzed by the ABDA interaction database). The risks were analyzed at two different time points: before and after start of cancer therapy. Severe toxicity during antineoplastic therapy was captured from medical records according to the Common Terminology Criteria for Adverse Events (CTCAE). The association between Grade ≥ 3 toxicity and medication risks was evaluated by univariate regression. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Part1 Gendiff.Qxp
Sex Differences in Health Status, Health Care Use, and Quality of Care: A Population-Based Analysis for Manitoba’s Regional Health Authorities November 2005 Manitoba Centre for Health Policy Department of Community Health Sciences Faculty of Medicine, University of Manitoba Randy Fransoo, MSc Patricia Martens, PhD The Need to KnowTeam (funded through CIHR) Elaine Burland, MSc Heather Prior, MSc Charles Burchill, MSc Dan Chateau, PhD Randy Walld, BSc, BComm (Hons) This report is produced and published by the Manitoba Centre for Health Policy (MCHP). It is also available in PDF format on our website at http://www.umanitoba.ca/centres/mchp/reports.htm Information concerning this report or any other report produced by MCHP can be obtained by contacting: Manitoba Centre for Health Policy Dept. of Community Health Sciences Faculty of Medicine, University of Manitoba 4th Floor, Room 408 727 McDermot Avenue Winnipeg, Manitoba, Canada R3E 3P5 Email: [email protected] Order line: (204) 789 3805 Reception: (204) 789 3819 Fax: (204) 789 3910 How to cite this report: Fransoo R, Martens P, The Need To Know Team (funded through CIHR), Burland E, Prior H, Burchill C, Chateau D, Walld R. Sex Differences in Health Status, Health Care Use and Quality of Care: A Population-Based Analysis for Manitoba’s Regional Health Authorities. Winnipeg, Manitoba Centre for Health Policy, November 2005. Legal Deposit: Manitoba Legislative Library National Library of Canada ISBN 1-896489-20-6 ©Manitoba Health This report may be reproduced, in whole or in part, provided the source is cited. 1st Printing 10/27/2005 THE MANITOBA CENTRE FOR HEALTH POLICY The Manitoba Centre for Health Policy (MCHP) is located within the Department of Community Health Sciences, Faculty of Medicine, University of Manitoba. -

Irish Medicines Board
Irish Medicines Board IRISH MEDICINES BOARD ACTS 1995 AND 2006 MEDICINAL PRODUCTS(CONTROL OF PLACING ON THE MARKET)REGULATIONS,2007 (S.I. No.540 of 2007) PPA1328/101/003 Case No: 2061333 The Irish Medicines Board in exercise of the powers conferred on it by the above mentioned Regulations hereby grants to B & S Healthcare Unit 4, Bradfield Road , Ruislip , Middlesex , HA4 0NU , United Kingdom an authorisation, subject to the provisions of the said Regulations, in respect of the product Tildiem LA 200mg Prolonged -Release Hard Capsules The particulars of which are set out in Part I and Part II of the attached Schedule. The authorisation is also subject to the general conditions as may be specified in the said Regulations as listed on the reverse of this document. This authorisation, unless previously revoked, shall continue in force from 08/05/2009 . Signed on behalf of the Irish Medicines Board this ________________ A person authorised in that behalf by the said Board. ______________________________________________________________________________________________________________________ Date Printed 12/05/2009 CRN 2061333 page number: 1 Irish Medicines Board Part II Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Tildiem LA 200 mg Prolonged -Release Hard Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 200mg diltiazem hydrochloride as the active ingredient in a combination of immediate release and coated prolonged -release pellets. For a full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Prolonged -release, hard capsules Product imported from the UK: Opaque capsules with a grey body and a pink cap containing white immediate release and prolonged -release pellets. -

Chapter 2 Methodological Aspects
Chapter 2 Methodological aspects Chapter 2.1 Indications for antihypertensive drug use in a prescription database BLG van Wijk, OH Klungel, ER Heerdink, A de Boer Department of Pharmacoepidemiology & Pharmacotherapy, Utrecht Institute for Pharmaceutical Sciences, Utrecht University Submitted CHAPTER 2.1 Summary Background: Drugs termed antihypertensives are prescribed and registered for other indications than hypertension alone. In pharmacoepidemiological studies, this could introduce several forms of bias. The objective of this study was to determine the extent to which drugs classified as antihypertensive drugs are prescribed for other diseases than hypertension. Methods: The NIVEL database was used, containing prescriptions from a sample of general practitioners in The Netherlands. Classes of antihypertensive drugs were analyzed separately, based on ATC-codes: miscellaneous antihypertensives, diuretics, beta-blockers, calcium channel blockers and agents acting on the renin angiotensin system. In addition, these classes were further subdivided based on their specific mechanism of action. All first prescriptions of a patient in the database for an antihypertensive drug were selected. ICPC diagnoses studied were: increased blood pressure, hypertension without organ damage, hypertension with organ damage and hypertension with diabetes mellitus for which the above- defined antihypertensive drugs were prescribed. Results: Of 24,812 patients who received a first prescription for an antihypertensive drug, 63.0% received a first prescription for hypertension related diagnoses (diuretics: 54.1%, beta-blockers: 59.1%, calcium channel blockers: 60.3%, agents acting on the renin angiotensin system: 82.8% and miscellaneous antihypertensives: 64.6%). Subdividing and restricting these subgroups based on their mechanism of action yields a higher percentage of first prescriptions with hypertension related diagnoses (low-ceiling diuretics: 78.8%, selective beta- blockers: 69.9% and dihydropyridine calcium channel blockers: 76.8%). -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P)