Cilazapril with Hydrochlorothiazide Will No Longer Be Available in New
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Apo-Cilazapril/Hydrochlorothiazide Film Coated Tablet
New Zealand Data Sheet APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE 1. PRODUCT NAME APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE – cilazapril 5mg and hydrochlorothiazide 12.5mg film coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Cilazapril monohydrate 5.22mg (equivalent to Cilazapril 5mg) and Hydrochlorothiazide 12.5mg Excipient(s) with known effect HYDROCHLOROTHIAZIDE contains sulphur. APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is lactose free and gluten free. APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE contains Red Ferric Oxide (orange shade # 34690). For the full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE are pink, oval biconvex film-coated tablets. Each tablet is engraved “APO” on one side and “5” bisect “12.5” on the other side. Each tablet typically weighs 92mg. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is indicated for the treatment of patients with hypertension who are not adequately controlled on monotherapy. 4.2 Dose and method of administration Standard Dosage The dosage of APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is one tablet administered once daily. As food intake has no clinically significant influence on absorption, APO- CILAZAPRIL/HYDROCHLOROTHIAZIDE can be administered before or after meals. The dose should always be taken at about the same time of day. Special Populations Renal insufficiency When concomitant diuretic therapy is required in patients with severe renal impairment, a loop diuretic rather than a thiazide diuretic is preferred for use with cilazapril/hydrochlorothiazide; therefore, for patients with severe renal dysfunction (creatinine Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 1 of 22 APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE clearance <10ml/min), APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is not recommended. -

Summary of Product Characteristics
Proposed var 24 psusa cilazapril SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [Fosinopril sodium 10 mg, tablets] [Fosinopril sodium 20 mg, tablets] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 or 20 mg fosinopril sodium. Excipient with known effect: Each tablet fosinopril sodium 10 mg contains 87 mg of lactose, anhydrous. Each tablet fosinopril sodium 20 mg contains 174 mg of lactose, anhydrous. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. The 10 mg tablets are white and shaped like a capsule with indents. On one side they are engraved with the letters “APO” and on the other side with “FOS-10”. The 20 mg tablets are white and their shape is oval. On one side they are engraved with the letters “APO” and on the other side with “FOS-20”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Treatment of hypertension. - Treatment of symptomatic heart failure. 4.2 Posology and method of administration Posology Fosinopril sodium should be administered orally in a single daily dose. As with all other medicinal products taken once daily, it should be taken at approximately the same time each day. The absorption of fosinopril sodium is not affected by food. The dose should be individualised according to patient profile and blood pressure response (see section 4.4). Hypertension: Fosinopril sodium may be used as a monotherapy or in combination with other classes of antihypertensive medicinal products (see section 4.3, 4.4, 4.5 and 5.1). Hypertensive patients not being treated with diuretics: Starting dose The initial recommended dose is 10 mg once a day. -

"Coaprovel, INN-Irbesartan+Hydrochlorothiazide"
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT CoAprovel 150 mg/12.5 mg tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 150 mg of irbesartan and 12.5 mg of hydrochlorothiazide. Excipient with known effect: Each tablet contains 26.65 mg of lactose (as lactose monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. Peach, biconvex, oval-shaped, with a heart debossed on one side and the number 2775 engraved on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of essential hypertension. This fixed dose combination is indicated in adult patients whose blood pressure is not adequately controlled on irbesartan or hydrochlorothiazide alone (see section 5.1). 4.2 Posology and method of administration Posology CoAprovel can be taken once daily, with or without food. Dose titration with the individual components (i.e. irbesartan and hydrochlorothiazide) may be recommended. When clinically appropriate direct change from monotherapy to the fixed combinations may be considered: . CoAprovel 150 mg/12.5 mg may be administered in patients whose blood pressure is not adequately controlled with hydrochlorothiazide or irbesartan 150 mg alone; . CoAprovel 300 mg/12.5 mg may be administered in patients insufficiently controlled by irbesartan 300 mg or by CoAprovel 150 mg/12.5 mg. CoAprovel 300 mg/25 mg may be administered in patients insufficiently controlled by CoAprovel 300 mg/12.5 mg. Doses higher than 300 mg irbesartan/25 mg hydrochlorothiazide once daily are not recommended. When necessary, CoAprovel may be administered with another antihypertensive medicinal product (see sections 4.3, 4.4, 4.5 and 5.1). -

Guidelines for the Management of Hyponatraemia in Hospitalised Patients
Guidelines for the management of hyponatraemia in hospitalised patients Authors: V Mishra Aims: To provide guidelines for appropriate investigations and treatment of hyponatraemia in hospitalised patients. Normal range Mild Moderate hyponatraemia Severe hyponatraemia hyponatraemia 135-146 mmol/L 130-135 mmol/L 120-129 mmol/L <120 mmol/L Evaluation of hyponatraemia STEP 1: Rule out artefactual causes Is the patient on IV fluids? If so, could the sample have been taken “downstream” from the infusion site? Could the sample have been taken from the same line? STEP 2: Clinical history Check fluid balance to exclude fluid overload Is the patient diabetic? Hyperglycemia may cause dilutional hyponatraemia and increased urinary loss of sodium Correct serum sodium for hyperglycemia (rise in plasma glucose >5.5mmol/L) by using equation given in (Appendix 1) Consider medications (Table 1). In some cases, stopping the medication or changing to an alternative that does not cause hyponatraemia may be sufficient. Monitor sodium concentrations to assess the effects of this management. It may take several days for the sodium to normalise after withdrawing medications Review clinical history for relevant conditions (such as congestive cardiac failure, kidney disease, liver failure, lung pathology) Loss of weight /appetite: investigate for malignancy 1 Table 1: Drugs known to cause hyponatraemia Drug group Examples known to causecause hyponatraemia (other compounds may exist) TThiazidehiazide diuretics Bendroflumethiazide, Metolazone, Indapamide, -

DIURETICS Diuretics Are Drugs That Promote the Output of Urine Excreted by the Kidneys
DIURETICS Diuretics are drugs that promote the output of urine excreted by the Kidneys. The primary action of most diuretics is the direct inhibition of Na+ transport at one or more of the four major anatomical sites along the nephron, where Na+ reabsorption takes place. The increased excretion of water and electrolytes by the kidneys is dependent on three different processes viz., glomerular filtration, tubular reabsorption (active and passive) and tubular secretion. Diuretics are very effective in the treatment of Cardiac oedema, specifically the one related with congestive heart failure. They are employed extensively in various types of disorders, for example, nephritic syndrome, diabetes insipidus, nutritional oedema, cirrhosis of the liver, hypertension, oedema of pregnancy and also to lower intraocular and cerebrospinal fluid pressure. Therapeutic Uses of Diuretics i) Congestive Heart Failure: The choice of the diuretic would depend on the severity of the disorder. In an emergency like acute pulmonary oedema, intravenous Furosemide or Sodium ethacrynate may be given. In less severe cases. Hydrochlorothiazide or Chlorthalidone may be used. Potassium-sparing diuretics like Spironolactone or Triamterene may be added to thiazide therapy. ii) Essential hypertension: The thiazides usually sever as primary antihypertensive agents. They may be used as sole agents in patients with mild hypertension or combined with other antihypertensives in more severe cases. iii) Hepatic cirrhosis: Potassium-sparing diuretics like Spironolactone may be employed. If Spironolactone alone fails, then a thiazide diuretic can be added cautiously. Furosemide or Ethacrymnic acid may have to be used if the oedema is regractory, together with spironolactone to lessen potassium loss. Serum potassium levels should be monitored periodically. -

Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research
Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Garabedian, Laura Faden. 2013. Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research. Doctoral dissertation, Harvard University. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:11156786 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research A dissertation presented by Laura Faden Garabedian to The Committee on Higher Degrees in Health Policy in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Health Policy Harvard University Cambridge, Massachusetts March 2013 © 2013 – Laura Faden Garabedian All rights reserved. Professor Stephen Soumerai Laura Faden Garabedian Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research Abstract This dissertation consists of two empirical papers and one methods paper. The first two papers use quasi-experimental methods to evaluate the impact of universal health insurance reform in Massachusetts (MA) and Thailand and the third paper evaluates the validity of a quasi- experimental method used in comparative effectiveness research (CER). My first paper uses interrupted time series with data from IMS Health to evaluate the impact of Thailand’s universal health insurance and physician payment reform on utilization of medicines for three non-communicable diseases: cancer, cardiovascular disease and diabetes. -
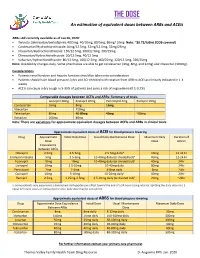
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Patient Information Leaflet
Package leaflet: Information for the patient Zornichka 12,5 mg tablets Zornichka 25 mg tablets Chlortalidone Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Zornichka is and what it is used for 2. What you need to know before you take Zornichka 3. How to take Zornichka 4. Possible side effects 5. How to store Zornichka 6. Contents of the pack and other information 1. What Zornichka is and what it is used for Zornichka belongs to a group of medicines called thiazide diuretics (“water tablets”). Thiazide diuretics help to reduce the amount of water in your body. They do this by increasing the amount of water that you pass as urine. Zornichka is used to: treat high blood pressure (hypertension) – as monotherapy or in combination with other antihypertensive (lowering high blood pressure) medicines. as an additional therapy for the treatment of edema caused by mild to moderate heart failure (II- III functional class); hepatic cirrhosis with ascites; corticosteroid and estrogen therapy, and some forms of impaired renal function (nephrotic syndrome, acute glomerulonephritis and chronic renal failure - creatinine clearance > 30 ml/min). -

Angiotensin-Converting Enzyme (ACE) Inhibitors
Angiotensin-Converting Enzyme (ACE) Inhibitors Summary Blood pressure reduction is similar for the ACE inhibitors class, with no clinically meaningful differences between agents. Side effects are infrequent with ACE inhibitors, and are usually mild in severity; the most commonly occurring include cough and hypotension. Captopril and lisinopril do not require hepatic conversion to active metabolites and may be preferred in patients with severe hepatic impairment. Captopril differs from other oral ACE inhibitors in its rapid onset and shorter duration of action, which requires it to be given 2-3 times per day; enalaprilat, an injectable ACE inhibitor also has a rapid onset and shorter duration of action. Pharmacology Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) block the conversion of angiotensin I to angiotensin II through competitive inhibition of the angiotensin converting enzyme. Angiotensin is formed via the renin-angiotensin-aldosterone system (RAAS), an enzymatic cascade that leads to the proteolytic cleavage of angiotensin I by ACEs to angiotensin II. RAAS impacts cardiovascular, renal and adrenal functions via the regulation of systemic blood pressure and electrolyte and fluid balance. Reduction in plasma levels of angiotensin II, a potent vasoconstrictor and negative feedback mediator for renin activity, by ACE inhibitors leads to increased plasma renin activity and decreased blood pressure, vasopressin secretion, sympathetic activation and cell growth. Decreases in plasma angiotensin II levels also results in a reduction in aldosterone secretion, with a subsequent decrease in sodium and water retention.[51035][51036][50907][51037][24005] ACE is found in both the plasma and tissue, but the concentration appears to be greater in tissue (primarily vascular endothelial cells, but also present in other organs including the heart). -
Choice of Medicines for Hypertension
CORRESPONDENCE Hypertension guidelines versus individual studies: Should we hang our hat on ALLHAT? Recommendations in Best Practice Journal are tailored to the needs of primary care health professionals by incorporating information from guidelines, and where necessary, adapting this to a New Zealand context. Naturally, this guidance will sometimes differ from conclusions that are based on individual studies. “Hypertension in Adults: The silent killer”, BPJ 54 (Aug, 2013) was largely based on the United Kingdom National Choice of medicines for hypertension Institute of Health and Care Excellence (NICE) guidelines Dear Editor for the clinical management of primary hypertension in There were a few problems with the article: “Hypertension in adults (2011).1 The discrepancies highlighted between the adults: the silent killer”, BPJ 54 (Aug, 2013). I usually find the bpacnz recommendations in the Best Practice Journal article and the resources well written and evidence based. In this review there results of the Antihypertensive and Lipid Lowering Treatment were a number of key errors: to Prevent Heart Attack Trial (ALLHAT) represent differences in 1. Start with an ACE inhibitor or calcium channel blocker. clinical/expert opinion rather than “key errors”. Ironically there is data that neither of these medications are more effective than chlorthalidone – a thiazide-like diuretic: 1. We agree that the ALLHAT trial published in 2002 did see ALLHAT study, JAMA 2002;288:2981-97, which found, not show evidence of superiority for thiazide-like diuretics albeit for the secondary but important outcome of combined over ACE inhibitors or calcium channel blockers. ALLHAT cardiovascular disease, that chlorthalidone was more effective reported that all three medicines were equally effective in 2 than lisinopril and amlodipine. -

Vascace Plus, INN-Cilazapril/Hydrochlorthiazid
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Vascace Plus and associated names (see Annex I) 5 mg/12.5 mg film-coated tablets [See Annex I - To be completed nationally] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains: 5.22 mg cilazapril equivalent to 5 mg cilazapril anhydrous and 12.5 mg hydrochlorothiazide Excipients with known effects: Each tablet contains 119.18 mg lactose monohydrate For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Pale red, oval, biconvex film-coated tablets with a score on one side and imprinted "CIL+" and underneath "5 + 12.5" on the other side. The tablet can be divided into equal doses. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Vascace Plus is indicated for the treatment of hypertension in adult patients whose blood pressure is not adequately controlled with cilazapril alone. 4.2 Posology and method of administration Posology Patients with renal impairment When concomitant diuretic therapy is required in patients with severe renal impairment, a loop diuretic rather than a thiazide diuretic is preferred for use with cilazapril. Therefore, Vascace Plus is not recommended for patients with severe renal impairment (see section 4.3). Patients with liver cirrhosis Because significant hypotension may occur in patients with liver cirrhosis treated with standard doses of ACE inhibitors, cautious dose titration of each individual component is needed if patients with liver cirrhosis should require treatment with cilazapril and hydrochlorothiazide (see section 4.4). Older people In clinical studies, the efficacy and tolerability of cilazapril and hydrochlorothiazide administered concomitantly was similar in both elderly and younger hypertensive patients, although pharmacokinetic data show that clearance of both components in elderly patients was reduced (see section 5.2). -

Therapeutic Combination and Use of Dll4 Antagonist Antibodies and Anti-Hypertensive Agents
(19) TZZ Z_T (11) EP 2 488 204 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 39/395 (2006.01) A61P 35/00 (2006.01) 06.04.2016 Bulletin 2016/14 A61P 9/12 (2006.01) (21) Application number: 10824244.7 (86) International application number: PCT/US2010/053064 (22) Date of filing: 18.10.2010 (87) International publication number: WO 2011/047383 (21.04.2011 Gazette 2011/16) (54) THERAPEUTIC COMBINATION AND USE OF DLL4 ANTAGONIST ANTIBODIES AND ANTI-HYPERTENSIVE AGENTS THERAPEUTISCHE KOMBINATION UND VERWENDUNGG MIT DLL4-ANTAGONISTISCHE ANTIKÖRPER UND MITTEL GEGEN BLUTHOCHDRUCK COMBINAISON THÉRAPEUTIQUE ET UTILISATION D’ANTICORPS ANTAGONISTES DE D LL4 ET D’AGENTS ANTI-HYPERTENSEURS (84) Designated Contracting States: US-A1- 2009 221 549 AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • SMITH D C ET AL: "222 A first-in-human, phase I PL PT RO RS SE SI SK SM TR trial of the anti-DLL4 antibody (OMP-21M18) targeting cancer stem cells (CSC) in patients with (30) Priority: 16.10.2009 US 252473 P advanced solid tumors", EUROPEAN JOURNAL OF CANCER. SUPPLEMENT, PERGAMON, (43) Date of publication of application: OXFORD, GB, vol. 8, no. 7, 1 November 2010 22.08.2012 Bulletin 2012/34 (2010-11-01), page 73, XP027497910, ISSN: 1359-6349, DOI: 10.1016/S1359-6349(10)71927-3 (60) Divisional application: [retrieved on 2010-11-01] & Smith et al: "A 16155324.3 first-in-human, phase I trial of the anti-DLL4 antibody (OMP-21M18) targeting cancer stem (73) Proprietor: Oncomed Pharmaceuticals, Inc.