"Coaprovel, INN-Irbesartan+Hydrochlorothiazide"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Appendix 1. Search Strategy for the Systematic Review and Meta-Analysis
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Thorax Supplementary Appendix 1. Search strategy for the systematic review and meta-analysis # COVID-19 AND (ACEI or ARB) Pubmed #1. COVID-19 ((((novel[Title/Abstract]) AND (((corona[Title/Abstract]) AND virus[Title/Abstract]) OR (coronavirus[Title/Abstract]))) OR ((COVID[Title/Abstract]) OR (COVID-19[Title/Abstract]) OR (nCoV[Title/Abstract]) OR (2019-nCoV[Title/Abstract]) OR (Novel Coronavirus Pneumon.ia[Title/Abstract]) OR (NCP[Title/Abstract]) OR (severe acute respiratory infection[Title/Abstract]) OR (SARI[Title/Abstract]) OR (SARS-CoV-2[Title/Abstract]))) #2. ARB (("Angiotensin Receptor Antagonists"[Mesh]) OR (((angiotensin receptor blocker[Title/Abstract]) OR angiotensin receptor blockers[Title/Abstract]) OR ARB.*[Title/Abstract]) OR (((angiotensin[Title/Abstract]) AND receptor[Title/Abstract]) AND (antagonist.*[Title/Abstract] OR inhibitor.*[Title/Abstract] OR blocker.*[Title/Abstract]))) OR (ARB[Title/Abstract]) OR (olmesartan[Title/Abstract]) OR (valsartan[Title/Abstract]) OR (eprosartan[Title/Abstract]) OR (irbesartan[Title/Abstract]) OR (candesartan[Title/Abstract]) OR (losartan[Title/Abstract]) OR (telmisartan[Title/Abstract]) OR (azilsartan[Title/Abstract]) OR (tasosartan[Title/Abstract]) OR (embusartan[Title/Abstract]) OR (forasartan[Title/Abstract]) OR (milfasartan[Title/Abstract]) OR (saprisartan[Title/Abstract]) OR (zolasartan[Title/Abstract]) -

Apo-Cilazapril/Hydrochlorothiazide Film Coated Tablet
New Zealand Data Sheet APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE 1. PRODUCT NAME APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE – cilazapril 5mg and hydrochlorothiazide 12.5mg film coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Cilazapril monohydrate 5.22mg (equivalent to Cilazapril 5mg) and Hydrochlorothiazide 12.5mg Excipient(s) with known effect HYDROCHLOROTHIAZIDE contains sulphur. APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is lactose free and gluten free. APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE contains Red Ferric Oxide (orange shade # 34690). For the full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE are pink, oval biconvex film-coated tablets. Each tablet is engraved “APO” on one side and “5” bisect “12.5” on the other side. Each tablet typically weighs 92mg. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is indicated for the treatment of patients with hypertension who are not adequately controlled on monotherapy. 4.2 Dose and method of administration Standard Dosage The dosage of APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is one tablet administered once daily. As food intake has no clinically significant influence on absorption, APO- CILAZAPRIL/HYDROCHLOROTHIAZIDE can be administered before or after meals. The dose should always be taken at about the same time of day. Special Populations Renal insufficiency When concomitant diuretic therapy is required in patients with severe renal impairment, a loop diuretic rather than a thiazide diuretic is preferred for use with cilazapril/hydrochlorothiazide; therefore, for patients with severe renal dysfunction (creatinine Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 1 of 22 APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE clearance <10ml/min), APO-CILAZAPRIL/HYDROCHLOROTHIAZIDE is not recommended. -

Summary of Product Characteristics
Proposed var 24 psusa cilazapril SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [Fosinopril sodium 10 mg, tablets] [Fosinopril sodium 20 mg, tablets] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 or 20 mg fosinopril sodium. Excipient with known effect: Each tablet fosinopril sodium 10 mg contains 87 mg of lactose, anhydrous. Each tablet fosinopril sodium 20 mg contains 174 mg of lactose, anhydrous. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. The 10 mg tablets are white and shaped like a capsule with indents. On one side they are engraved with the letters “APO” and on the other side with “FOS-10”. The 20 mg tablets are white and their shape is oval. On one side they are engraved with the letters “APO” and on the other side with “FOS-20”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Treatment of hypertension. - Treatment of symptomatic heart failure. 4.2 Posology and method of administration Posology Fosinopril sodium should be administered orally in a single daily dose. As with all other medicinal products taken once daily, it should be taken at approximately the same time each day. The absorption of fosinopril sodium is not affected by food. The dose should be individualised according to patient profile and blood pressure response (see section 4.4). Hypertension: Fosinopril sodium may be used as a monotherapy or in combination with other classes of antihypertensive medicinal products (see section 4.3, 4.4, 4.5 and 5.1). Hypertensive patients not being treated with diuretics: Starting dose The initial recommended dose is 10 mg once a day. -

Ace Inhibitors (Angiotensin-Converting Enzyme)
Medication Instructions Ace Inhibitors (Angiotensin-Converting Enzyme) Generic Brand Benazepril Lotensin Captopril Capoten Enalapril Vasotec Fosinopril Monopril Lisinopril Prinivil, Zestril Do not Moexipril Univasc Quinapril Accupril stop taking Ramipril Altace this medicine Trandolapril Mavik About this Medicine unless told ACE inhibitors are used to treat both high blood pressure (hypertension) and heart failure (HF). They block an enzyme that causes blood vessels to constrict. This to do so allows the blood vessels to relax and dilate. Untreated, high blood pressure can damage to your heart, kidneys and may lead to stroke or heart failure. In HF, using by your an ACE inhibitor can: • Protect your heart from further injury doctor. • Improve your health • Reduce your symptoms • Can prevent heart failure. Generic forms of ACE Inhibitors (benazepril, captopril, enalapril, fosinopril, and lisinopril) may be purchased at a lower price. There are no “generics” for Accupril, Altace Mavik, and of Univasc. Thus their prices are higher. Ask your doctor if one of the generic ACE Inhibitors would work for you. How to Take Use this drug as directed by your doctor. It is best to take these drugs, especially captopril, on an empty stomach one hour before or two hours after meals (unless otherwise instructed by your doctor). Side Effects Along with needed effects, a drug may cause some unwanted effects. Many people will not have any side effects. Most of these side effects are mild and short-lived. Check with your doctor if any of the following side effects occur: • Fever and chills • Hoarseness • Swelling of face, mouth, hands or feet or any trouble in swallowing or breathing • Dizziness or lightheadedness (often a problem with the first dose) Report these side effects if they persist: • Cough – dry or continuing • Loss of taste, diarrhea, nausea, headache or unusual fatigue • Fast or irregular heartbeat, dizziness, lightheadedness • Skin rash Special Guidelines • Sodium in the diet may cause you to retain fluid and increase your blood pressure. -

Hypertension and Cardiac Arrhythmias: a Review of the Epidemiology, Pathophysiology and Clinical Implications
Journal of Human Hypertension (2008) 22, 380–388 & 2008 Nature Publishing Group All rights reserved 0950-9240/08 $30.00 www.nature.com/jhh REVIEW Hypertension and cardiac arrhythmias: a review of the epidemiology, pathophysiology and clinical implications K-H Yiu and H-F Tse Division of Cardiology, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong, China Hypertension is commonly associated with cardiac cular ectopy and sudden cardiac death. Recent arrhythmias in patients with and without concomitant prospective clinical trials reveal that antihyper- cardiovascular disease. Experimental and epidemiolo- tensive therapy may delay or prevent the occurrence gical studies have demonstrated potential links of cardiac arrhythmias and sudden cardiac death in between hypertension and atrial and ventricular arrhyth- patients with hypertension. Although antihypertensive mias, although the underlying pathophysiological me- agents that block the renin–angiotensin–aldosterone chanism remains unclear. Nonetheless, the importance system appear to protect against cardiac arrhythmias, of hypertension as a cause of atrial and ventricular this needs to be confirmed by current ongoing clinical arrhythmias is not well recognized. In particular, trials. the occurrence of left ventricular hypertrophy is a Journal of Human Hypertension (2008) 22, 380–388; strong predictor for the development of AF, ventri- doi:10.1038/jhh.2008.10; published online 13 March 2008 Keywords: atrial fibrillation; arrhythmia; sudden cardiac death Introduction Epidemiology Concomitant cardiac arrhythmias are commonly Atrial fibrillation seen in patients with hypertension, although the Atrial fibrillation is the most common sustained mechanism of this association is unclear. The arrhythmia in adults and is associated with an contribution of hypertension to the development of increased risk for cardiovascular morbidity, mortality atrial and ventricular arrhythmias is unrecognized and stroke.1,2 The incidence of AF increases with age; and thus undertreated. -

“Captopril” [Mesh
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Open Heart PubMed: 1. (“dose comparison” OR “dose” OR “low dose” OR “high dose” OR “dosage*”) 2. (“captopril” [mesh] OR “Benazepril” OR “Enalapril” [mesh] OR “Enalapril” OR “Cilazapril” [mesh] OR “Cilazapril” OR “Delapril” OR “Imidapril” OR “Lisinopril” [mesh] OR “Lisinopril” OR “Moexipril” OR “Perindopril” [mesh] OR “Perindopril” OR “Quinapril” OR “Ramipril” [mesh] “Ramipril” OR “Spirapril” OR “Temocapril” OR “Trandolapril” OR “Zofenopril” OR “Enalaprilat” [mesh] OR “Enalaprilat” OR “Fosinopril” [mesh] OR “Fosinopril” OR “Teprotide” [mesh] OR “Teprotide” OR “Angiotensin-Converting Enzyme Inhibitors” [mesh] OR “Angiotensin-Converting Enzyme Inhibitors” OR “Angiotensin Converting Enzyme Inhibitors” OR “Inhibitors, Kininase II” OR “Kininase II Antagonists” OR “Kininase II Inhibitors” OR “Angiotensin I-Converting Enzyme Inhibitors” OR “Angiotensin I Converting Enzyme Inhibitors” OR “Antagonists, Angiotensin- Converting Enzyme” OR “Antagonists, Angiotensin Converting Enzyme” OR “Antagonists, Kininase II” OR “Inhibitors, ACE” OR “ACE Inhibitors” OR “Inhibitors, Angiotensin-Converting Enzyme” OR “Enzyme Inhibitors, Angiotensin-Converting” OR “Inhibitors, Angiotensin Converting Enzyme” OR “Angiotensin-Converting Enzyme Antagonists” OR “Angiotensin Converting Enzyme Antagonists” OR “Enzyme Antagonists, Angiotensin-Converting”) 3. (“Heart failure” [mesh] OR “heart failure” OR “Cardiac Failure” OR “Heart Decompensation” OR “Decompensation, Heart” OR “Heart Failure, Right-Sided” OR “Heart Failure, Right Sided” OR “Right-Sided Heart Failure” OR “Right Sided Heart Failure” OR “Myocardial Failure” OR “Congestive Heart Failure” OR “Heart Failure, Congestive” OR “Heart Failure, Left-Sided” OR “Heart Failure, Left Sided” OR “Left-Sided Heart Failure” OR “Left Sided Heart Failure” OR “chronic heart failure” OR "chronic heart" OR (CHF)) 4. -

Angioedema After Long-Term Use of an Angiotensin-Converting Enzyme Inhibitor
J Am Board Fam Pract: first published as 10.3122/jabfm.10.5.370 on 1 September 1997. Downloaded from BRIEF REPORTS Angioedema After Long-Term Use of an Angiotensin-Converting Enzyme Inhibitor Adriana]. Pavietic, MD Angioedema is an uncommon adverse effect of cyclobenzaprine, her angioedema was initially be angiotensin-converting enzyme (ACE) inhib lieved to be an allergic reaction to this drug, and itors. Its frequency ranges from 0.1 percent in pa it was discontinued. She was also given 125 mg of tients on captopril, lisinopril, and quinapril to 0.5 methylprednisolone intramuscularly. Her an percent in patients on benazepril. l Most cases are gioedema did not improve, and she returned to mild and occur within the first week of treat the clinic the following day. She was seen by an ment. 2-4 Recent reports indicate that late-onset other physician, who discontinued lisinopril, and angioedema might be more prevalent than ini her symptoms resolved within 24 hours. Mter her tially thought, and fatal cases have been de ACE inhibitor was discontinued, she experienced scribed.5-11 Many physicians are not familiar with more symptoms and an increased frequency of late-onset angioedema associated with ACE in palpitations, but she had no change in exercise hibitors, and a delayed diagnosis can have poten tolerance. A cardiologist was consulted, who pre tially serious consequences.5,8-II scribed the angiotensin II receptor antagonist losartan (initially at dosages of 25 mg/d and then Case Report 50 mg/d). The patient was advised to report any A 57-year-old African-American woman with symptoms or signs of angioedema immediately copyright. -
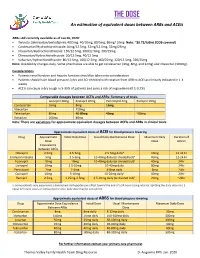
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Angiotensin-Converting Enzyme (ACE) Inhibitors
Angiotensin-Converting Enzyme (ACE) Inhibitors Summary Blood pressure reduction is similar for the ACE inhibitors class, with no clinically meaningful differences between agents. Side effects are infrequent with ACE inhibitors, and are usually mild in severity; the most commonly occurring include cough and hypotension. Captopril and lisinopril do not require hepatic conversion to active metabolites and may be preferred in patients with severe hepatic impairment. Captopril differs from other oral ACE inhibitors in its rapid onset and shorter duration of action, which requires it to be given 2-3 times per day; enalaprilat, an injectable ACE inhibitor also has a rapid onset and shorter duration of action. Pharmacology Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) block the conversion of angiotensin I to angiotensin II through competitive inhibition of the angiotensin converting enzyme. Angiotensin is formed via the renin-angiotensin-aldosterone system (RAAS), an enzymatic cascade that leads to the proteolytic cleavage of angiotensin I by ACEs to angiotensin II. RAAS impacts cardiovascular, renal and adrenal functions via the regulation of systemic blood pressure and electrolyte and fluid balance. Reduction in plasma levels of angiotensin II, a potent vasoconstrictor and negative feedback mediator for renin activity, by ACE inhibitors leads to increased plasma renin activity and decreased blood pressure, vasopressin secretion, sympathetic activation and cell growth. Decreases in plasma angiotensin II levels also results in a reduction in aldosterone secretion, with a subsequent decrease in sodium and water retention.[51035][51036][50907][51037][24005] ACE is found in both the plasma and tissue, but the concentration appears to be greater in tissue (primarily vascular endothelial cells, but also present in other organs including the heart). -
![5 Mg Film-Coated Tablets [Nationally Completed Name] 10 Mg Film-Coated Tablets [Nationally Completed Name] 20 Mg Film-Coated Tablets](https://docslib.b-cdn.net/cover/3029/5-mg-film-coated-tablets-nationally-completed-name-10-mg-film-coated-tablets-nationally-completed-name-20-mg-film-coated-tablets-1833029.webp)
5 Mg Film-Coated Tablets [Nationally Completed Name] 10 Mg Film-Coated Tablets [Nationally Completed Name] 20 Mg Film-Coated Tablets
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [nationally completed name] 5 mg film-coated tablets [nationally completed name] 10 mg film-coated tablets [nationally completed name] 20 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains 5/10/20 mg Benazepril hydrochloride. For the full list of excipients see section 6.1 3. PHARMACEUTICAL FORM Film-coated tablet [nationally completed name] 5 mg film-coated tablets: Oval (4 x 8 mm), light yellow film-coated tablets with score on both sides. [nationally completed name] 10 mg film-coated tablets: Oval (11 x 5.5 mm) yellow film-coated tablets with score on both sides. [nationally completed name] 20 mg film-coated tablets: Oval (11 x 5.5 mm) light red film-coated tablet with score on both sides. The tablet can be divided into equal doses. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Essential hypertension, congestive heart failure - in addition to diuretics and especially to digitalis glycosides in cases of severe congestive heart failure 4.2 Posology and method of administration Posology Paediatric population [nationally completed name] is not recommended for use in children due to insufficient data on safety and efficacy (see section 4.4). Essential hypertension 10 to 20 mg daily divided in one or two doses. Maximum daily dose is 40 mg. Congestive heart failure Initially 2.5 mg daily. After 2-4 weeks the dosage can be increased to 5 mg daily. Maximum dose is 20 mg daily. Dosage at renal impairment For the treatment of essential hypertension the dose should be reduced if creatinine clearance is below 30 ml/min. -

Lotensin® (Benazepril Hydrochloride) Tablets Rx Only Prescribing Information
® Lotensin (benazepril hydrochloride) Tablets Rx only Prescribing Information WARNING: FETAL TOXICITY When pregnancy is detected, discontinue Lotensin as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity DESCRIPTION Benazepril hydrochloride is a white to off-white crystalline powder, soluble (>100 mg/mL) in water, in ethanol, and in methanol. Its chemical name is benazepril 3-[[1-(ethoxy-carbonyl)-3-phenyl-(1S) propyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benzazepine-1-acetic acid monohydrochloride; its structural formula is Its empirical formula is C24H28N2O5•HCl, and its molecular weight is 460.96. Benazeprilat, the active metabolite of benazepril, is a non-sulfhydryl angiotensin-converting enzyme inhibitor. Benazepril is converted to benazeprilat by hepatic cleavage of the ester group. Lotensin is supplied as tablets containing 5 mg, 10 mg, 20 mg, and 40 mg of benazepril hydrochloride for oral administration. The inactive ingredients are colloidal silicon dioxide, crospovidone, hydrogenated castor oil (5-mg, 10-mg, and 20-mg tablets), hypromellose, iron oxides, lactose, magnesium stearate (40-mg tablets), microcrystalline cellulose, polysorbate 80, propylene glycol (5-mg and 40-mg tablets), starch, talc, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Benazepril and benazeprilat inhibit angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. Reference ID: 3692061 Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. -

BENAZEPRIL HYDROCHLORIDE- Benazepril Hydrochloride Tablet DIRECT RX ------BENAZEPRIL HYDROCHLORIDE
BENAZEPRIL HYDROCHLORIDE- benazepril hydrochloride tablet DIRECT RX ---------- BENAZEPRIL HYDROCHLORIDE BOXED WARNING SECTION WARNING: FETAL TOXICITY When pregnancy is detected, discontinue benazepril hydrochloride as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity DESCRIPTION SECTION Benazepril hydrochloride (HCl), USP is a white to off-white crystalline powder, soluble (>100 mg/mL) in water, in ethanol, and in methanol. Its chemical name is benazepril 3-[[1-(ethoxy-carbonyl)-3-phenyl- (1S)-propyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benzazepine-1-acetic acid monohydrochloride; its structural formula is Its empirical formula is C24H28N2O5•HCl, and its molecular weight is 460.96. Benazeprilat, the active metabolite of benazepril, is a non-sulfhydryl angiotensin-converting enzyme inhibitor. Benazepril is converted to benazeprilat by hepatic cleavage of the ester group. Benazepril HCl tablets, USP are supplied as white, round, biconvex tablets containing either 5 mg, 10 mg, 20 mg, or 40 mg of benazepril HCl, USP for oral administration. The inactive ingredients are crospovidone, lactose anhydrous, magnesium stearate, microcrystalline cellulose, pregelatinized corn starch and talc. CLINICAL PHARMACOLOGY SECTION Mechanism of Action Benazepril and benazeprilat inhibit angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium.