Apo-Cilazapril/Hydrochlorothiazide Film Coated Tablet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Product Characteristics
Proposed var 24 psusa cilazapril SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [Fosinopril sodium 10 mg, tablets] [Fosinopril sodium 20 mg, tablets] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 or 20 mg fosinopril sodium. Excipient with known effect: Each tablet fosinopril sodium 10 mg contains 87 mg of lactose, anhydrous. Each tablet fosinopril sodium 20 mg contains 174 mg of lactose, anhydrous. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. The 10 mg tablets are white and shaped like a capsule with indents. On one side they are engraved with the letters “APO” and on the other side with “FOS-10”. The 20 mg tablets are white and their shape is oval. On one side they are engraved with the letters “APO” and on the other side with “FOS-20”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Treatment of hypertension. - Treatment of symptomatic heart failure. 4.2 Posology and method of administration Posology Fosinopril sodium should be administered orally in a single daily dose. As with all other medicinal products taken once daily, it should be taken at approximately the same time each day. The absorption of fosinopril sodium is not affected by food. The dose should be individualised according to patient profile and blood pressure response (see section 4.4). Hypertension: Fosinopril sodium may be used as a monotherapy or in combination with other classes of antihypertensive medicinal products (see section 4.3, 4.4, 4.5 and 5.1). Hypertensive patients not being treated with diuretics: Starting dose The initial recommended dose is 10 mg once a day. -

"Coaprovel, INN-Irbesartan+Hydrochlorothiazide"
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT CoAprovel 150 mg/12.5 mg tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 150 mg of irbesartan and 12.5 mg of hydrochlorothiazide. Excipient with known effect: Each tablet contains 26.65 mg of lactose (as lactose monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. Peach, biconvex, oval-shaped, with a heart debossed on one side and the number 2775 engraved on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of essential hypertension. This fixed dose combination is indicated in adult patients whose blood pressure is not adequately controlled on irbesartan or hydrochlorothiazide alone (see section 5.1). 4.2 Posology and method of administration Posology CoAprovel can be taken once daily, with or without food. Dose titration with the individual components (i.e. irbesartan and hydrochlorothiazide) may be recommended. When clinically appropriate direct change from monotherapy to the fixed combinations may be considered: . CoAprovel 150 mg/12.5 mg may be administered in patients whose blood pressure is not adequately controlled with hydrochlorothiazide or irbesartan 150 mg alone; . CoAprovel 300 mg/12.5 mg may be administered in patients insufficiently controlled by irbesartan 300 mg or by CoAprovel 150 mg/12.5 mg. CoAprovel 300 mg/25 mg may be administered in patients insufficiently controlled by CoAprovel 300 mg/12.5 mg. Doses higher than 300 mg irbesartan/25 mg hydrochlorothiazide once daily are not recommended. When necessary, CoAprovel may be administered with another antihypertensive medicinal product (see sections 4.3, 4.4, 4.5 and 5.1). -
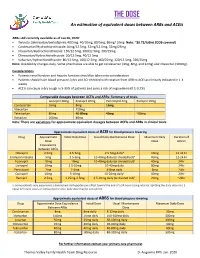
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Angiotensin-Converting Enzyme (ACE) Inhibitors
Angiotensin-Converting Enzyme (ACE) Inhibitors Summary Blood pressure reduction is similar for the ACE inhibitors class, with no clinically meaningful differences between agents. Side effects are infrequent with ACE inhibitors, and are usually mild in severity; the most commonly occurring include cough and hypotension. Captopril and lisinopril do not require hepatic conversion to active metabolites and may be preferred in patients with severe hepatic impairment. Captopril differs from other oral ACE inhibitors in its rapid onset and shorter duration of action, which requires it to be given 2-3 times per day; enalaprilat, an injectable ACE inhibitor also has a rapid onset and shorter duration of action. Pharmacology Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) block the conversion of angiotensin I to angiotensin II through competitive inhibition of the angiotensin converting enzyme. Angiotensin is formed via the renin-angiotensin-aldosterone system (RAAS), an enzymatic cascade that leads to the proteolytic cleavage of angiotensin I by ACEs to angiotensin II. RAAS impacts cardiovascular, renal and adrenal functions via the regulation of systemic blood pressure and electrolyte and fluid balance. Reduction in plasma levels of angiotensin II, a potent vasoconstrictor and negative feedback mediator for renin activity, by ACE inhibitors leads to increased plasma renin activity and decreased blood pressure, vasopressin secretion, sympathetic activation and cell growth. Decreases in plasma angiotensin II levels also results in a reduction in aldosterone secretion, with a subsequent decrease in sodium and water retention.[51035][51036][50907][51037][24005] ACE is found in both the plasma and tissue, but the concentration appears to be greater in tissue (primarily vascular endothelial cells, but also present in other organs including the heart). -

Vascace Plus, INN-Cilazapril/Hydrochlorthiazid
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Vascace Plus and associated names (see Annex I) 5 mg/12.5 mg film-coated tablets [See Annex I - To be completed nationally] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains: 5.22 mg cilazapril equivalent to 5 mg cilazapril anhydrous and 12.5 mg hydrochlorothiazide Excipients with known effects: Each tablet contains 119.18 mg lactose monohydrate For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Pale red, oval, biconvex film-coated tablets with a score on one side and imprinted "CIL+" and underneath "5 + 12.5" on the other side. The tablet can be divided into equal doses. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Vascace Plus is indicated for the treatment of hypertension in adult patients whose blood pressure is not adequately controlled with cilazapril alone. 4.2 Posology and method of administration Posology Patients with renal impairment When concomitant diuretic therapy is required in patients with severe renal impairment, a loop diuretic rather than a thiazide diuretic is preferred for use with cilazapril. Therefore, Vascace Plus is not recommended for patients with severe renal impairment (see section 4.3). Patients with liver cirrhosis Because significant hypotension may occur in patients with liver cirrhosis treated with standard doses of ACE inhibitors, cautious dose titration of each individual component is needed if patients with liver cirrhosis should require treatment with cilazapril and hydrochlorothiazide (see section 4.4). Older people In clinical studies, the efficacy and tolerability of cilazapril and hydrochlorothiazide administered concomitantly was similar in both elderly and younger hypertensive patients, although pharmacokinetic data show that clearance of both components in elderly patients was reduced (see section 5.2). -

Cilazapril with Hydrochlorothiazide Will No Longer Be Available in New
CARDIOVASCULAR SYSTEM MEDICINE SUBSIDY News Update: cilazapril with hydrochlorothiazide will no longer be available in New Zealand Patients currently prescribed cilazapril + hydrochlorothiazide combination tablets will need to transition to a different combination medicine or antihypertensive regimen. Cilazapril with hydrochlorothiazide is a fixed-dose combination – Patients still receiving this combination product medicine (cilazapril 5mg + hydrochlorothiazide 12.5 mg) after 1 March, 2020 will need to have switched to an for the treatment of patients with hypertension when dual alternative regimen by July, 2020 antihypertensive treatment is indicated.1 Apotex, the supplier * Pharmacists may annotate a prescription of cilazapril with of Apo-Cilazapril/Hydrochlorothiazide, has announced that it hydrochlorothiazide as endorsed if a record exists of prior dispensing, if 2 will no longer be able to supply this medicine in New Zealand. required. Apo-Cilazapril/Hydrochlorothiazide is the only registered brand of cilazapril with hydrochlorothiazide in New Zealand, Deciding which antihypertensive regimen to therefore this decision will mean that: switch to From 1 March, 2020: cilazapril with hydrochlorothiazide will no longer be available funded for patients that have Patients with hypertension currently taking cilazapril with never been prescribed this medicine before hydrochlorothiazide will need to transition to an alternative antihypertensive regimen; the decision on the replacement – Prescribers should ensure no new patients are started regimen should be individualised. Most patients are likely on this medicine from 1 March, 2020 to prefer to continue using another fixed-dose combination – Prescribers will need to endorse* any prescriptions because they have established a familiar dosing routine, and for patients who were taking this medicine prior to 1 it is likely to have a comparable clinical effect. -

Apo-Cilazapril/Hydrochlorothiazide Cilazapril 5Mg + Hydrochlorothiazide 12.5Mg Film Coated Tablets
New Zealand Consumer Medicine Information Apo-Cilazapril/Hydrochlorothiazide cilazapril 5mg + hydrochlorothiazide 12.5mg film coated tablets What is in this leaflet Please read this leaflet carefully before you start using Apo-Cilazapril/Hydrochlorothiazide. This leaflet answers some common questions about Apo-Cilazapril/Hydrochlorothiazide. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist. All medicines have risks and benefits. Your doctor has weighed the risks of you using Apo- Cilazapril/Hydrochlorothiazide against the benefits they expect it will have for you. If you have any concerns about using this medicine, ask your doctor or pharmacist. Keep this leaflet with the medicine. You may need to read it again. What Apo-Cilazapril/Hydrochlorothiazide is used for Apo-Cilazapril/Hydrochlorothiazide contains two active ingredients, cilazapril and hydrochlorothiazide. Cilazapril belongs to a group of medicines known as ACE (Angiotensin Converting Enzyme) inhibitors. Hydrochlorothiazide belongs to a group of medicines known as thiazide diuretics. Cilazapril and hydrochlorothiazide are both used to treat raised blood pressure. When used in combination they are more effective at lowering blood pressure than either medicine used alone. Cilazapril works by inhibiting (blocking) natural chemicals produced by the body which increase blood pressure. This inhibition leads to a reduction of blood pressure. When given together with diuretics (fluid tablets) this helps to reduce the blood pressure further. Your doctor may have prescribed Apo-Cilazapril/Hydrochlorothiazide for another reason. Ask your doctor if you have any questions about why Apo- Cilazapril/Hydrochlorothiazide has been prescribed for you. This medicine is available only with a doctor's prescription. -

1.3.1 Cilazapril SPC, Labeling and Package Leaflet CZ
1.3.1 Cilazapril SPC, Labeling and Package Leaflet CZ SUMMARY OF PRODUCT CHARACTERISTICS, LABELLING AND PACKAGE LEAFLET SmPCPIL124891_2 15.01.2019 – Updated: 12.03.2019 Page 1 of 28 1.3.1 Cilazapril SPC, Labeling and Package Leaflet CZ SUMMARY OF PRODUCT CHARACTERISTICS SmPCPIL124891_2 15.01.2019 – Updated: 12.03.2019 Page 2 of 28 1.3.1 Cilazapril SPC, Labeling and Package Leaflet CZ 1. NAME OF THE MEDICINAL PRODUCT <Invented name> 0.5 mg film-coated tablets <Invented name> 1 mg film-coated tablets <Invented name> 2.5 mg film-coated tablets <Invented name> 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION <Invented name> 0.5 mg: Each film-coated tablet contains cilazapril monohydrate 0.522 mg, which corresponds to cilazapril 0.5 mg. <Invented name> 1 mg: Each film-coated tablet contains cilazapril monohydrate 1.044 mg, which corresponds to cilazapril 1.0 mg. <Invented name> 2.5 mg: Each film-coated tablet contains cilazapril monohydrate 2.61 mg, which corresponds to cilazapril 2.5 mg. <Invented name> 5 mg: Each film-coated tablet contains cilazapril monohydrate 5.22 mg, which corresponds to cilazapril 5.0 mg. Excipient(s) with known effect: <Invented name> 0.5 mg: Each film-coated tablet contains 107.86 mg lactose. <Invented name> 1 mg: Each film-coated tablet contains 107.36 mg lactose. <Invented name> 2.5 mg: Each film-coated tablet contains 162.89 mg lactose. <Invented name> 5 mg: Each film-coated tablet contains 160.41 mg lactose. For the full list of excipients, see section 6.1. -

Angiotensin Converting Enzyme Inhibitors (Aceis) (PDF)
Modernized Reference Drug Program Angiotensin Converting Enzyme Inhibitors (ACEIs) Fully Covered (Reference Drugs) Partially Covered (Non-Reference Drugs) • Ramipril with/without • Benazepril • Enalapril maleate or sodium with/without HCTZ • Perindopril Hydrochlorothiazide (HCTZ) • Captopril • Fosinopril • Quinapril with/without HCTZ • Cilazapril with/without HCTZ • Lisinopril with/without HCTZ • Trandolapril Information provided is not intended as a substitute for professional judgement. Step 1 – Does your patient need to switch medications to retain PharmaCare coverage? Is patient already taking the fully covered (reference) drug listed above? NO YES No medication change Pharmacists To confirm Special Authority Is patient concerned about prescription costs and about getting Prescribers coverage for patient’s the most PharmaCare coverage possible? To confirm Special Authority current medication, call coverage for the patient’s the PharmaCare HelpDesk and select the YES NO No medication change current medication, call 1-866-905-4912 Self-Service Option Does patient already have Special Authority coverage of their existing drug (i.e., is eligible for continued full coverage as explained in Section 4 of the Guide to the Modernized RDP)? NO YES No medication change Prescribers Does the patient meet the criteria (below) for full coverage of a drug Submit a Pharmacists that will be only partially covered as of December 1, 2016? Special Authority Request If the patient meets the Specialty Exemptions from criteria, refer to prescriber Criteria for full coverage of a partially covered (non-reference) drug* submitting Special Authority who can submit a Treatment failure on optimal doses or intolerance to ramipril or Requests: paediatric Special Authority Request Complex patient requiring medication(s) for co-existing chronic condition(s) cardiology and paediatrics for coverage NO YES Step 2 – Making the switch Consider the following precautions: Pharmacists • Be aware that captopril is dosed multiple times per day, whereas other ACEIs are typically dosed once daily. -

Review of Existing Classification Efforts
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.1.1 Review of existing classification efforts Due date of deliverable: (15.01.2008) Actual submission date: (07.02.2008) Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UGent Revision 1.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public X PP Restricted to other programme participants (including the Commission Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) Task 4.1 : Review of existing classification efforts Authors: Kristof Pil, Elke Raes, Thomas Van den Neste, An-Sofie Goessaert, Jolien Veramme, Alain Verstraete (Ghent University, Belgium) Partners: - F. Javier Alvarez (work package leader), M. Trinidad Gómez-Talegón, Inmaculada Fierro (University of Valladolid, Spain) - Monica Colas, Juan Carlos Gonzalez-Luque (DGT, Spain) - Han de Gier, Sylvia Hummel, Sholeh Mobaser (University of Groningen, the Netherlands) - Martina Albrecht, Michael Heiβing (Bundesanstalt für Straßenwesen, Germany) - Michel Mallaret, Charles Mercier-Guyon (University of Grenoble, Centre Regional de Pharmacovigilance, France) - Vassilis Papakostopoulos, Villy Portouli, Andriani Mousadakou (Centre for Research and Technology Hellas, Greece) DRUID 6th Framework Programme Deliverable D.4.1.1. Revision 1.0 Review of Existing Classification Efforts Page 2 of 127 Introduction DRUID work package 4 focusses on the classification and labeling of medicinal drugs according to their influence on driving performance. -

Apo-Perindopril, Tablet
NEW ZEALAND DATASHEET APO-PERINDOPRIL This product may not be interchangeable with similar products on the New Zealand market. 1. APO-PERINDOPRIL(2mg, 4mg and 8mg tablets) 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Perindopril erbumine 2mg, 4mg and 8mg Excipient(s) of known effect APO-PERINDOPRIL contain Lactose. APO-PERINDOPRIL are gluten free. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM APO-PERINDOPRIL 2mg tablets are white, round, biconvex tablets identified by an engraved “APO” on one side and “PE2” on the reverse. APO-PERINDOPRIL 4mg tablets are white, capsule-shaped, biconvex tablets identified by an engraved “APO” on one side and “PE” bisect “4” on the reverse. APO-PERINDOPRIL 8mg tablets are white, capsule-shaped, biconvex tablets identified by an engraved “APO” on one side and “PE” bisect “8” on the reverse. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications APO-PERINDOPRIL is indicated for: • the treatment of hypertension; • the treatment of heart failure. In such patients it is recommended that APO- PERINDOPRIL be given with a diuretic and/or digoxin under close medical supervision. (The safety and efficacy of APO-PERINDOPRIL has not been demonstrated for New York Heart Association Category IV patients); • Reduction of risk of cardiovascular events (cardiovascular mortality, myocardial infarction or cardiac arrest) in patients with established coronary artery disease who are stable on concomitant therapy. 4.2 Dose and method of administration Food intake may reduce hepatic biotransformation of perindopril to perindoprilat. However, whilst this effect has not been shown to be clinically significant, it is recommended that APO-PERINDOPRIL should be taken before meals. -

Part IB Summary of Product Characteristics
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT <INVENTED NAM E > 2.5 mg, film-coated tablets <INVENTED NAM E > 5 mg, film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mg film-coated tablet contains 2.5 mg of cilazapril (as cilazapril monohydrate). Excipient(s) with known effect: Each 2.5 mg film-coated tablet contains 58.912 mg of lactose monohydrate Each 5 mg film-coated tablet contains 5 mg of cilazapril (as cilazapril monohydrate). Excipient(s) with known effect: Each 5 mg film-coated tablet contains 117.824 mg of lactose monohydrate For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Film-coated tablet. Pink, oblong presenting a one sided score, weighing approximately 100 mg, film coated tablets. Pink, oblong presenting a one sided score, with the mark C1 engraved on one side, weighing approximately 200 mg, film coated tablets. Brown, oblong presenting a one sided score, film coated tablets Brown, oblong presenting a one sided score, with the mark C5 engraved on one side, weighing approximately 200 mg, film coated tablets The tablet can be divided into equal doses. 4 CLINICAL PARTICULARS 4.1 Therapeutic indications <invented name> is indicated for the treatment of hypertension. <invented name> is indicated for the treatment of chronic heart failure. 4.2 Posology and method of administration Posology <invented name> should be administered once daily. As food intake has no clinically significant influence on absorption, <invented name> can be administered before or after a meal. The dose should always be taken at about the same time of day.