Drugs and Life-Threatening Ventricular Arrhythmia Risk: Results from the DARE Study Cohort
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -

WHO Drug Information Vol
WHO Drug Information Vol. 31, No. 3, 2017 WHO Drug Information Contents Medicines regulation 420 Post-market monitoring EMA platform gains trade mark; Automated 387 Regulatory systems in India FDA field alert reports 421 GMP compliance Indian manufacturers to submit self- WHO prequalification certification 421 Collaboration 402 Prequalification process quality China Food and Drug Administration improvement initiatives: 2010–2016 joins ICH; U.S.-EU cooperation in inspections; IGDRP, IPRF initiatives to join 422 Medicines labels Safety news Improved labelling in Australia 423 Under discussion 409 Safety warnings 425 Approved Brimonidine gel ; Lactose-containing L-glutamine ; Betrixaban ; C1 esterase injectable methylprednisolone inhibitor (human) ; Meropenem and ; Amoxicillin; Azithromycin ; Fluconazole, vaborbactam ; Delafloxacin ; Glecaprevir fosfluconazole ; DAAs and warfarin and pibrentasvir ; Sofosbuvir, velpatasvir ; Bendamustine ; Nivolumab ; Nivolumab, and voxilaprevir ; Cladribine ; Daunorubicin pembrolizumab ; Atezolizumab ; Ibrutinib and cytarabine ; Gemtuzumab ozogamicin ; Daclizumab ; Loxoprofen topical ; Enasidenib ; Neratinib ; Tivozanib ; preparations ; Denosumab ; Gabapentin Guselkumab ; Benznidazole ; Ciclosporin ; Hydroxocobalamine antidote kit paediatric eye drops ; Lutetium oxodotreotide 414 Diagnostics Gene cell therapy Hightop HIV home testing kits Tisagenlecleucel 414 Known risks Biosimilars Warfarin ; Local corticosteroids Bevacizumab; Adalimumab ; Hydroquinone skin lighteners Early access 415 Review outcomes Idebenone -
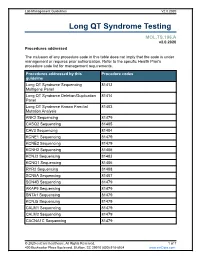
Long QT Syndrome Testing
Lab Management Guidelines V2.0.2020 Long QT Syndrome Testing MOL.TS.196.A v2.0.2020 Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline Long QT Syndrome Sequencing 81413 Multigene Panel Long QT Syndrome Deletion/Duplication 81414 Panel Long QT Syndrome Known Familial 81403 Mutation Analysis ANK2 Sequencing 81479 CASQ2 Sequencing 81405 CAV3 Sequencing 81404 KCNE1 Sequencing 81479 KCNE2 Sequencing 81479 KCNH2 Sequencing 81406 KCNJ2 Sequencing 81403 KCNQ1 Sequencing 81406 RYR2 Sequencing 81408 SCN5A Sequencing 81407 SCN4B Sequencing 81479 AKAP9 Sequencing 81479 SNTA1 Sequencing 81479 KCNJ5 Sequencing 81479 CALM1 Sequencing 81479 CALM2 Sequencing 81479 CACNA1C Sequencing 81479 © 2020 eviCore healthcare. All Rights Reserved. 1 of 7 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V2.0.2020 What is Long QT syndrome Definition Long QT Syndrome (LQTS) is caused by mutations in a number of genes, most of which are related to the functioning of sodium or potassium ion channels in the heart.1 Testing may offer prognostic information in some cases, as specific genes and even specific mutations within those genes may have some correlation to risk for sudden death, effectiveness of beta-blocker therapy, and preventive strategies.1-4 Signs and symptoms of long QT syndrome (LQTS) are variable, but may include a prolonged QT interval on an electrocardiogram, torsades de pointes, syncope, seizures, cardiac arrest, and sudden cardiac death.1,2 Many patients with LQTS can be largely asymptomatic, with cardiac arrest or sudden cardiac death as the first and only symptom. -

Weak Interaction of the Antimetabolite Drug Methotrexate with a Cavitand Derivative
International Journal of Molecular Sciences Article Weak Interaction of the Antimetabolite Drug Methotrexate with a Cavitand Derivative Zsolt Preisz 1,2, Zoltán Nagymihály 3,4, Beáta Lemli 1,4 ,László Kollár 3,4 and Sándor Kunsági-Máté 1,2,4,* 1 Institute of Organic and Medicinal Chemistry, Medical School, University of Pécs, Szigeti 12, H-7624 Pécs, Hungary; [email protected] (Z.P.); [email protected] (B.L.) 2 Department of General and Physical Chemistry, Faculty of Sciences, University of Pécs, Ifjúság 6, H 7624 Pécs, Hungary 3 Department of Inorganic Chemistry, Faculty of Sciences, University of Pécs, Ifjúság 6, H 7624 Pécs, Hungary; [email protected] (Z.N.); [email protected] (L.K.) 4 János Szentágothai Research Center, University of Pécs, Ifjúság 20, H-7624 Pécs, Hungary * Correspondence: [email protected]; Tel.: +36-72-503600 (ext. 35449) Received: 2 June 2020; Accepted: 16 June 2020; Published: 18 June 2020 Abstract: Formation of inclusion complexes involving a cavitand derivative (as host) and an antimetabolite drug, methotrexate (as guest) was investigated by photoluminescence measurements in dimethyl sulfoxide solvent. Molecular modeling performed in gas phase reflects that, due to the structural reasons, the cavitand can include the methotrexate in two forms: either by its opened structure with free androsta-4-en-3-one-17α-ethinyl arms or by the closed form when all the androsta-4-en-3-one-17α-ethinyl arms play role in the complex formation. Experiments reflect enthalpy driven complex formation in higher temperature range while at lower temperature the complexes are stabilized by the entropy gain. -

Public Assessment Report
Public Assessment Report Tifix 150mg and 500mg film-coated tablets Capecitabine BE/H/195/01-02/DC BE licence no: BE423552-BE423561 Applicant: Alfred E. Tiefenbacher, Germany Date: 27-04-2012 Toepassingsdatum : 15-09-10 Page 1 of 17 Blz. 1 van 17 This assessment report is published by the Federal Agency for Medicines and Health Products following Article 21 (3) and (4) of Directive 2001/83/EC, amended by Directive 2004/27/EC and Article 25 paragraph 4 of Directive 2001/82/EC as amended by 2004/28/EC. The report comments on the registration dossier that was submitted to the Federal Agency for Medicines and Health Products and its fellow organisations in all concerned EU member states. It reflects the scientific conclusion reached by the Federal Agency for Medicines and Health Products and all concerned member states at the end of the evaluation process and provides a summary of the grounds for approval of a marketing authorisation. This report is intended for all those involved with the safe and proper use of the medicinal product, i.e. healthcare professionals, patients and their family and carers. Some knowledge of medicines and diseases is expected of the latter category as the language in this report may be difficult for laymen to understand. This assessment report shall be updated by a following addendum whenever new information becomes available. To the best of the Federal Agency for Medicines and Health Products’ knowledge, this report does not contain any information that should not have been made available to the public. The Marketing Autorisation Holder has checked this report for the absence of any confidential information. -

Hepatic Arterial Infusion for Unresectable Colorectal Liver Metastases Combined Or Not with Systemic Chemotherapy
ANTICANCER RESEARCH 29: 4139-4144 (2009) Hepatic Arterial Infusion for Unresectable Colorectal Liver Metastases Combined or Not with Systemic Chemotherapy PIERLUIGI PILATI, ENZO MAMMANO, SIMONE MOCELLIN, EMANUELA TESSARI, MARIO LISE and DONATO NITTI Clinica Chirurgica II, Department of Oncological and Surgical Sciences, University of Padova, 35128 Padova, Italy Abstract. Background: The hypothesis was tested that CRC have liver metastases at autopsy, and the majority of systemic chemotherapy might contribute to improving overall these patients die as a result of their metastatic disease. survival (OS) of patients with unresectable colorectal liver Surgical resection represents the standard treatment of metastases treated with hepatic arterial infusion (HAI). resectable disease and is followed by 5-year overall Patients and Methods: We considered 153 consecutive patients survival (OS) rates of 20% to 40% (2, 3). Unfortunately, retrospectively divided into group A (n=72) treated with HAI only 20% of patients with liver metastases from CRC alone (floxuridine [FUDR] + leucovorin [LV]), and group B present with liver-confined resectable disease and/or are (n=81) treated with HAI combined with systemic candidates for major surgical operation (depending on chemotherapy (5-fluorouracil [5FU] + LV). Results: No comorbidities) (4, 5). significant difference in OS was observed between the two The therapeutic management of unresectable metastases is groups. Median OS was better in patients with <50% of liver more controversial and is generally associated with a dismal involvement (21.3 vs. 13.2 months; p<0.0001) and in prognosis. In fact, despite the improvements achieved with responders vs. non-responders (24.4 vs. 13.4 months; modern systemic chemotherapy (SCT) regimens (e.g. -

Atrial Fibrillation (ATRIA) Study
European Journal of Human Genetics (2014) 22, 297–306 & 2014 Macmillan Publishers Limited All rights reserved 1018-4813/14 www.nature.com/ejhg REVIEW Atrial fibrillation: the role of common and rare genetic variants Morten S Olesen*,1,2,4, Morten W Nielsen1,2,4, Stig Haunsø1,2,3 and Jesper H Svendsen1,2,3 Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting 1–2% of the general population. A number of studies have demonstrated that AF, and in particular lone AF, has a substantial genetic component. Monogenic mutations in lone and familial AF, although rare, have been recognized for many years. Presently, mutations in 25 genes have been associated with AF. However, the complexity of monogenic AF is illustrated by the recent finding that both gain- and loss-of-function mutations in the same gene can cause AF. Genome-wide association studies (GWAS) have indicated that common single-nucleotide polymorphisms (SNPs) have a role in the development of AF. Following the first GWAS discovering the association between PITX2 and AF, several new GWAS reports have identified SNPs associated with susceptibility of AF. To date, nine SNPs have been associated with AF. The exact biological pathways involving these SNPs and the development of AF are now starting to be elucidated. Since the first GWAS, the number of papers concerning the genetic basis of AF has increased drastically and the majority of these papers are for the first time included in a review. In this review, we discuss the genetic basis of AF and the role of both common and rare genetic variants in the susceptibility of developing AF. -

Should Genetic Testing Be Recommended for Long QT Syndrome Patients and Their Relatives?
The Science Journal of the Lander College of Arts and Sciences Volume 11 Number 1 Fall 2017 - 2017 Should Genetic Testing Be Recommended for Long QT Syndrome Patients and Their Relatives? Menachem Braun Touro College Follow this and additional works at: https://touroscholar.touro.edu/sjlcas Part of the Cardiovascular Diseases Commons, and the Medical Genetics Commons Recommended Citation Braun, M. (2017). Should Genetic Testing Be Recommended for Long QT Syndrome Patients and Their Relatives?. The Science Journal of the Lander College of Arts and Sciences, 11(1). Retrieved from https://touroscholar.touro.edu/sjlcas/vol11/iss1/8 This Article is brought to you for free and open access by the Lander College of Arts and Sciences at Touro Scholar. It has been accepted for inclusion in The Science Journal of the Lander College of Arts and Sciences by an authorized editor of Touro Scholar. For more information, please contact [email protected]. Should Genetic Testing be Recommended for Long QT Syndrome Patients and Their Relatives? Menachem Braun Menachem Braun graduated with a BS in Biology in September 2017. Abstract 7KH/RQJ476\QGURPH /476 LVDIDPLOLDOSRWHQWLDOO\IDWDOFDUGLDFDUUK\WKPLD7UDGLWLRQDOO\LWKDVEHHQGLDJQRVHGE\(&* Molecular studies have provided evidence that LQTS can be caused by a range of underlying molecular abnormalities. Genetic research has proven that different forms of LQTS have different genotypic bases. Therefore, it has become possible to diagnose WKHVSHFLÀFW\SHRIGLVHDVHJHQHWLFDOO\7KLVVWXG\H[DPLQHVWKHDGYDQFHPHQWVPDGHLQWKHSDVWWKLUW\\HDUVLQXQGHUVWDQGLQJ /476DQGUHVHDUFKUHJDUGLQJWKHXVHRIJHQHWLFWHVWLQJLQRUGHUWRGHWHUPLQHWKHEHQHÀWVRIJHQHWLFWHVWLQJIRUWKLVGLVHDVH$ survey of original studies which produced the information is presented here, and provides the reader with an understanding of the PHFKDQLFVRIWKHGLVHDVHDQGKRZWKH\GLIIHULQWKHVHYHUDOJHQHWLFYDULDQWV5HVHDUFKVKRZVWKDWWKHEHQHÀWRIJHQHWLFWHVWLQJ must be weighed against the personal implications in may have for a particular patient and his or her family. -

DIURETICS Diuretics Are Drugs That Promote the Output of Urine Excreted by the Kidneys
DIURETICS Diuretics are drugs that promote the output of urine excreted by the Kidneys. The primary action of most diuretics is the direct inhibition of Na+ transport at one or more of the four major anatomical sites along the nephron, where Na+ reabsorption takes place. The increased excretion of water and electrolytes by the kidneys is dependent on three different processes viz., glomerular filtration, tubular reabsorption (active and passive) and tubular secretion. Diuretics are very effective in the treatment of Cardiac oedema, specifically the one related with congestive heart failure. They are employed extensively in various types of disorders, for example, nephritic syndrome, diabetes insipidus, nutritional oedema, cirrhosis of the liver, hypertension, oedema of pregnancy and also to lower intraocular and cerebrospinal fluid pressure. Therapeutic Uses of Diuretics i) Congestive Heart Failure: The choice of the diuretic would depend on the severity of the disorder. In an emergency like acute pulmonary oedema, intravenous Furosemide or Sodium ethacrynate may be given. In less severe cases. Hydrochlorothiazide or Chlorthalidone may be used. Potassium-sparing diuretics like Spironolactone or Triamterene may be added to thiazide therapy. ii) Essential hypertension: The thiazides usually sever as primary antihypertensive agents. They may be used as sole agents in patients with mild hypertension or combined with other antihypertensives in more severe cases. iii) Hepatic cirrhosis: Potassium-sparing diuretics like Spironolactone may be employed. If Spironolactone alone fails, then a thiazide diuretic can be added cautiously. Furosemide or Ethacrymnic acid may have to be used if the oedema is regractory, together with spironolactone to lessen potassium loss. Serum potassium levels should be monitored periodically. -

An Electrocardiographic Series of Flecainide Toxicity
Indian Pacing and Electrophysiology Journal xxx (xxxx) xxx Contents lists available at ScienceDirect Indian Pacing and Electrophysiology Journal journal homepage: www.elsevier.com/locate/IPEJ An electrocardiographic series of flecainide toxicity * Alexandra Smith , Gregg Gerasimon San Antonio Military Medical Center, Electrophysiology Division, Cardiology Section, 3551 Roger Brooke Drive, San Antonio, TX, 78234, USA article info abstract Article history: Anti-arrhythmic drugs (AADs) uniquely affect the various electrolyte channels in the heart and can slow Received 16 October 2018 conduction, increase refractoriness, and/or decrease automaticity with the goal of preventing tachyar- Received in revised form rhythmias. Due to these properties, these same drugs are by nature pro-arrhythmic. Vaughan-Williams 8 November 2018 classification Ic AADs belong to a class of medications that inhibit sodium channels, leading to decreased Accepted 27 November 2018 conduction velocity of myocytes and Purkinje fibers as well as to decreased automaticity of pacemaker Available online xxx cells. When present in toxic amounts, this leads to classic changes on the electrocardiogram (ECG) that are harbingers of potentially lethal arrhythmias. Presented is a clinical series of ECGs that occurred in a Keywords: fl Flecainide patient who presented with ecainide toxicity. © Antiarrhythmic drugs Copyright 2018, Indian Heart Rhythm Society. Production and hosting by Elsevier B.V. This is an open Toxicity access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Atrial fibrillation Flecainide toxicity can lead to bradycardia, sinoatrial block, and Abbreviations asystole, as well as to first and second degree atrioventricular block. Sinus bradycardia is more common in patients with pre-existing AAD Antiarrhythmic drug sinus node dysfunction [2]. -

Heart Failure — Managing Newly Diagnosed and Decompensated
Clinical Guideline Heart failure: Managing newly diagnosed and decompensated patients admitted to hospital 1. Confirmation of Diagnosis Person with signs and symptoms suggesting heart failure Detailed history and clinical examination Consider aetiology for new diagnosis of heart Suspected diagnosis Confirmed failure or underlying cause for exacerbation of of heart failure diagnosis of heart chronic heart failure and exclude treatable Diagnosis has not failure causes. been confirmed by Diagnosis confirmed Arrange other investigations: echocardiogram by previous . CXR echocardiogram . ECG . FBC . U&Es and Creatinine . LFTs . TFTs . RBG . Cholesterol If current echocardiogram not Diagnosis confirmed by clinically relevant, echocardiogram, if possible Heart failure excluded so request repeat performed as an in-patient review diagnosis echo, if possible and adhering to Advancing performed as an in- Quality heart failure (AQHF) patient indicators Heart failure with preserved ejection Heart failure due to significant left ventricular fraction / diastolic dysfunction (EF systolic dysfunction (EF < 40%) >55%) • Update primary diagnosis on PCIS / -Update primary diagnosis on PCIS Cerner and document in casenotes /Cerner and document in casenotes. Proceed to Management of Confirmed Heart Failure Heart failure — managing newly diagnosed and decompensated patients in acute care — clinical guidelines, v1 Principal author: Dr P Saravanan Approved by Medicines Clinical Guideline Team: July 2013 Review by: July 2016 Page 1 of 27 2. Inpatient management Heart failure with preserved Heart failure due to left ventricular ejection fraction systolic dysfunction (EF < 40%) • Referral to heart failure specialist team. • Arrange admission to appropriate ward/unit Refer to Heart Failure Fluid balance: Drug management: Specialist Nurse for 1. Fluid restriction 1-2 litres in 24 1. -

Amiodarone-Induced Torsade De Pointes in a Patient with Wolff
Hellenic J Cardiol 2009; 50: 224-226 Case Report Amiodarone-Induced Torsade de Pointes in a Patient with Wolff-Parkinson-White Syndrome 1 1 1,2 3 AAREF BADSHAH , BAKHTIAR MIRZA , MUHAMMAD JANJUA , RAJIV NAIR , 3 3 RUSSELL T. STEINMAN , JOHN F. COTANT 1Department of Internal Medicine, Saint Joseph Mercy-Oakland Hospital, Pontiac, Michigan, 2Department of Internal Medicine, William Beaumont Hospital, Royal Oak, Michigan, 3Department of Cardiology, Saint Joseph Mercy-Oakland Hospital, Pontiac, Michigan, USA Key words: Amiodarone is generally regarded to have a high safety profile with a low incidence of arrhythmias. However, Atrial fibrillation, there have been reports of torsades de pointes under certain conditions, such as electrolyte imbalance or amiodarone, T-wave alternans, Wolff- concomitant antiarrhythmic therapy. We describe a case of amiodarone-induced torsade de pointes early Parkinson-White after initiation of intravenous amiodarone in the setting of T-wave alternans. syndrome, torsade de pointes. miodarone is generally regarded to mate total dose of 1 g), sinus rhythm was have a high safety profile with a low restored. The analysis of the ECG upon A incidence of arrhythmias. How- cardioversion revealed a short PR interval ever, there have been reports of torsades with QT interval prolongation (QTm 582 de pointes under certain conditions, such as ms, QTc 582 ms) with evidence of pre-ex- Manuscript received: electrolyte imbalance or concomitant an- citation (delta waves) in the precordial December 25, 2008; tiarrhythmic therapy. We describe a case of leads (Figure 2) and macroscopic T-wave Accepted: amiodarone-induced torsade de pointes ear- alternans. In light of his electrocardiogra- March 3, 2009.