Laboratory Safety Plan
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -
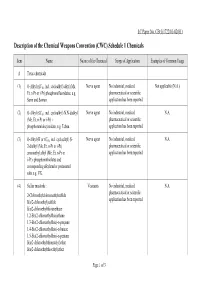
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

Alcoholsalcohols
AlcoholsAlcohols Chapter 10 1 Structure of Alcohols • The functional group of an alcohol is H an -OH group bonded to an sp3 O 108.9° hybridized carbon. C H – Bond angles about the hydroxyl oxygen H H atom are approximately 109.5°. • Oxygen is sp3 hybridized. –Two sp3 hybrid orbitals form sigma bonds to a carbon and a hydrogen. – The remaining two sp3 hybrid orbitals each contain an unshared pair of electrons. 2 Nomenclature of Alcohols • IUPAC names – The parent chain is the longest chain that contains the OH group. – Number the parent chain to give the OH group the lowest possible number. – Change the suffix -etoe -ol.ol • Common names – Name the alkyl group bonded to oxygen followed by the word alcohol.alcohol 3 Nomenclature of Alcohols OH o o 1o OH 1 2 OH 1-Propanol 2-Propan ol 1-Bu tanol (Pro py l alco ho l) (Isoprop yl alcoh ol) (Bu tyl alcoh ol) OH 2o OH 1o OH 3o 2-Butanol 2-M eth yl-1-p ropan ol 2-M eth yl-2-p ropan ol (sec-Butyl alcohol) (Isobutyl alcohol) (tert -Butyl alcohol) 4 Nomenclature of Alcohols • Compounds containing more than one OH group are named diols, triols, etc. CH2 CH2 CH3 CHCH2 CH2 CHCH2 OH OH HO OH HO HO OH 1,2-Ethanediol 1,2-Propanediol 1,2,3-Propanetriol (Ethylene glycol) (Propylene glycol) (Glycerol, Glycerine) 5 Physical Properties • Alcohols are polar compounds. – They interact with themselves and with other polar compounds by dipole-dipole interactions. • Dipole-dipole interaction: The attraction between the positive end of one dipole and the negative end of another. -

Development and Applications of Novel
DEVELOPMENT AND APPLICATIONS OF NOVEL SOLVENT-MINIMIZED TECHNIQUES IN THE DETERMINATION OF CHEMICAL WARFARE AGENTS AND THEIR DEGRADATION PRODUCTS LEE HOI SIM NANCY NATIONAL UNIVERSITY OF SINGAPORE 2008 DEVELOPMENT AND APPLICATIONS OF NOVEL SOLVENT-MINIMIZED TECHNIQUES IN THE DETERMINATION OF CHEMICAL WARFARE AGENTS AND THEIR DEGRADATION PRODUCTS LEE HOI SIM NANCY (M.Sc.), NUS A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF CHEMISTRY NATIONAL UNIVERSITY OF SINGAPORE 2008 ACKNOWLEDGEMENTS My most sincere gratitude goes to my bosses at DSO National Laboratories, Ms. Sng Mui Tiang, Dr. Lee Fook Kay and Assoc. Prof. Lionel Lee Kim Hock, who gave me the opportunity to pursue a part-time higher degree and provided me with much encouragement and support throughout the course of my study. I had the privilege of working under the expert guidance of Prof. Lee Hian Kee and Dr. Chanbasha Basheer of the Department of Chemistry at the National University of Singapore and would like to thank them for this enriching learning experience as well as their friendship over the years. I sincerely appreciate the help and support from everyone at the Defense Medical and Environmental Research Institute, DSO National Laboratories, especially from the Organic Synthesis Group for providing the analytes used in the study and all users of the GC MSD for sharing the use of the instrument. Even though people come and go, the memories of the exciting times especially during the Proficiency Tests, with Dr. Ang Kiam Wee, Ms. Chan Shu Cheng, Mr. Leonard Chay Yew Leong, Dr. Alex Chin Piao, Dr. Chua Guan Leong, Ms. -

Table of Contents
UNIVERSITY OF MISSOURI-KANSAS CITY CHEMICAL MANAGEMENT PLAN Revised May 2016 UMKC CHEMICAL MANAGEMENT PLAN This document constitutes the Chemical Management Plan (CMP) for the University of Missouri-Kansas City (UMKC). It was developed by the Environmental Health and Safety Department (EHS), to ensure the safe and proper use of hazardous and non- hazardous chemicals and to comply with applicable governmental regulations addressing the disposal of these chemicals. In addition, it was developed to foster waste minimization, and to provide the faculty and the staff with a management program to reduce the potential for accidents involving hazardous chemicals and/or wastes. Elements of the CMP include: a. a procedure for identifying potential or actual hazardous chemicals or wastes b. a procedure for periodic reexamination of those hazardous chemicals or wastes identified by the procedure in (a.) above as well as a systematic method for identification and evaluation of any new potential or actual hazardous chemicals or wastes c. procedures for labeling, and inventorying hazardous chemicals or wastes d. a procedure for identification and training of personnel directly responsible for ensuring that (a.), (b.), and (c.) are implemented e. a procedure for monitoring, recording, and reporting compliance with the CMP f. a procedure by which information generated by the CMP is provided to the persons performing waste analyses Each element is addressed as part of the complete CMP in the following paragraphs. 4 Table of Contents 1 Definitions 7 2 Identification -

Acetylcholinesterase: the “Hub” for Neurodegenerative Diseases And
Review biomolecules Acetylcholinesterase: The “Hub” for NeurodegenerativeReview Diseases and Chemical Weapons Acetylcholinesterase: The “Hub” for Convention Neurodegenerative Diseases and Chemical WeaponsSamir F. de A. Cavalcante Convention 1,2,3,*, Alessandro B. C. Simas 2,*, Marcos C. Barcellos 1, Victor G. M. de Oliveira 1, Roberto B. Sousa 1, Paulo A. de M. Cabral 1 and Kamil Kuča 3,*and Tanos C. C. França 3,4,* Samir F. de A. Cavalcante 1,2,3,* , Alessandro B. C. Simas 2,*, Marcos C. Barcellos 1, Victor1 Institute G. M. ofde Chemical, Oliveira Biological,1, Roberto Radiological B. Sousa and1, Paulo Nuclear A. Defense de M. Cabral (IDQBRN),1, Kamil Brazilian Kuˇca Army3,* and TanosTechnological C. C. França Center3,4,* (CTEx), Avenida das Américas 28705, Rio de Janeiro 23020-470, Brazil; [email protected] (M.C.B.); [email protected] (V.G.M.d.O.); [email protected] 1 Institute of Chemical, Biological, Radiological and Nuclear Defense (IDQBRN), Brazilian Army (R.B.S.); [email protected] (P.A.d.M.C.) Technological Center (CTEx), Avenida das Américas 28705, Rio de Janeiro 23020-470, Brazil; 2 [email protected] Mors Institute of Research (M.C.B.); on Natural [email protected] Products (IPPN), Federal (V.G.M.d.O.); University of Rio de Janeiro (UFRJ), CCS,[email protected] Bloco H, Rio de Janeiro (R.B.S.); 21941-902, [email protected] Brazil (P.A.d.M.C.) 32 DepartmentWalter Mors of Institute Chemistry, of Research Faculty of on Science, Natural Un Productsiversity (IPPN), -

Department of Homeland Security (Dhs) Appendix a Chemicals of Interest (Coi)
DEPARTMENT OF HOMELAND SECURITY (DHS) APPENDIX A CHEMICALS OF INTEREST (COI) Acetaldehyde Bromine trifluoride Acetone Cyanohydrin, stabilized Bromothrifluorethylene Acetyl bromide 1,3-Butadiene Acetyl chloride Butane Acetyl iodide Butene Acetylene 1-Butene Acrolein 2-Butene Acrylonitrile 2-Butene-cis Acrylyl chloride 2-Butene-trans Allyl alcohol Butyltrichlorosilane Allylamine Calcium hydrosulfite Allyltrichlorosilane, stabilized Calcium phosphide Aluminum (powder) Carbon disulfide Aluminum Bromide, anhydrous Carbon oxysulfide Aluminum Chloride, anhydrous Carbonyl fluoride Aluminum phosphide Carbonyl sulfide Ammonia (anhydrous) Chlorine Ammonia (conc 20% or greater) Chlorine dioxide Ammonium nitrate, note #1 Chlorine monoxide Ammonium nitrate, note #2 Chlorine pentafluoride Ammonium perchlorate Chlorine trifluoride Ammonium picrate Chloroacetyl chloride Amyltrichlorosilane 2-Chloroethylchloro-methylsulfide Antimony pentafluoride Chloroform Arsenic trichloride Chloromethyl ether Arsine Chloromethyl methyl ether Barium azide 1-Chloropropylene 1,4-Bis(2-chloroethylthio)-n-butane 2-Chloropropylene Bis(2-chloroethylthio) methane Chlorosarin Bis(2-chloroethylthiomethyl)ether Chlorosoman 1,5-Bis(2-chloroethylthio)-n-pentane Chlorosulfonic acid 1,3-Bis(2-chloroethylthio)-n-propane Chromium oxychloride Boron tribromide Crotonaldehyde Boron trichloride Crotonaldehyde, (E)- Boron trifluoride Cyanogen Boron trifluoride compound with methyl Cyanogen chloride ether (1:1) Cyclohexylamine Bromine Cyclohexyltrichlorosilane Bromine chloride Cyclopropane -

Manganese-Catalyzed Β‑Alkylation of Secondary Alcohols with Primary Alcohols Under Phosphine-Free Conditions
Letter Cite This: ACS Catal. 2018, 8, 7201−7207 pubs.acs.org/acscatalysis Manganese-Catalyzed β‑Alkylation of Secondary Alcohols with Primary Alcohols under Phosphine-Free Conditions † ‡ † † † § Tingting Liu, , Liandi Wang, Kaikai Wu, and Zhengkun Yu*, , † Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, P. R. China ‡ University of Chinese Academy of Sciences, Beijing 100049, P. R. China § State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Road, Shanghai 200032, P. R. China *S Supporting Information ABSTRACT: Manganese(I) complexes bearing a pyridyl-supported pyr- azolyl-imidazolyl ligand efficiently catalyzed the direct β-alkylation of secondary alcohols with primary alcohols under phosphine-free conditions. The β-alkylated secondary alcohols were obtained in moderate to good yields with water formed as the byproduct through a borrowing hydrogen pathway. β-Alkylation of cholesterols was also effectively achieved. The present protocol provides a concise atom-economical method for C−C bond formation from primary and secondary alcohols. KEYWORDS: manganese, alcohols, alkylation, borrowing hydrogen, cholesterols onstruction of carbon−carbon bonds is of great impor- metals. In particular, it is capable of existing in several C tance in organic synthesis.1 More and more concern on oxidation states. Milstein and co-workers reported α- the consequence of climate change and dwindling crude oil olefination of nitriles,14a dehydrogenative cross-coupling of reserves results in the search for alternative carbon resources alcohols with amines,14b N-formylation of amines with 2 for the formation of C−C bonds. Thus, readily available methanol,15 and deoxygenation of alcohols16 catalyzed by alcohols have recently been paid much attention to be utilized pincer-type Mn-PNP complexes. -

Export Control Handbook for Chemicals
Export Control Handbook for Chemicals -Dual-use control list -Common Military List -Explosives precursors -Syria restrictive list -Psychotropics and narcotics precursors ARNES-NOVAU, X 2019 EUR 29879 This publication is a Technical report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aims to provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use that might be made of this publication. Contact information Xavier Arnés-Novau Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-785421 Filippo Sevini Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-786793 EU Science Hub https://ec.europa.eu/jrc JRC 117839 EUR 29879 Print ISBN 978-92-76-11971-5 ISSN 1018-5593 doi:10.2760/844026 PDF ISBN 978-92-76-11970-8 ISSN 1831-9424 doi:10.2760/339232 Luxembourg: Publications Office of the European Union, 2019 © European Atomic Energy Community, 2019 The reuse policy of the European Commission is implemented by Commission Decision 2011/833/EU of 12 December 2011 on the reuse of Commission documents (OJ L 330, 14.12.2011, p. 39). Reuse is authorised, provided the source of the document is acknowledged and its original meaning or message is not distorted. The European Commission shall not be liable for any consequence stemming from the reuse. -
![Phosphine Solubilities 281 1. Phosphine; PH3; 2. Organic Liquids. [7803-51-2] P. G. T. Fogg, School of Chemistry, Polytechnic Of](https://docslib.b-cdn.net/cover/1173/phosphine-solubilities-281-1-phosphine-ph3-2-organic-liquids-7803-51-2-p-g-t-fogg-school-of-chemistry-polytechnic-of-2051173.webp)
Phosphine Solubilities 281 1. Phosphine; PH3; 2. Organic Liquids. [7803-51-2] P. G. T. Fogg, School of Chemistry, Polytechnic Of
Phosphine Solubilities 281 COHPONENTS: EVALUATOR: P. G. T. Fogg, 1. Phosphine; PH ; [7803-51-2] School of Chemistry, 3 Polytechnic of North London, 2. Organic liquids. Holloway, London N7 8DB United Kingdom. August 1983 CRITICAL EVALUATION: Data obtained by Palmer et aZ (1) and by Devyatykh et aZ (2) have been discussed in detail by Gerrard (3). Mole fraction solubilities calculated from measurements by Palmer et aZ. fall into a consistent pattern with a lower solubility in hydrogen bonded solvents compared with other solvents. There is also an increase in mole fraction solubility with increase in chain length in the case of straight chain alkanes. Solubilities are in the order: in benzene < in cyclohexene < in cyclohexane and in benzene < in toluene < in xylene. The variation of solubility in nitrobenzene, with change in temperature, was measured by both Palmer et aZ. and by Devyatykh et aZ. The ratio V01'PH3/vOl'solvent for a temperature of 295.2 K has been estimated by the compiler from data given by Devyatykh et aZ. to be 8.4. This may be compared with the value of 3.06 given by Palmer et aZ. for a pressure of 1 atm. There is a similar discrepancy between the solubility in liquid paraffin calculated from the data of Devyatykh et aZ and that measured by Palmer. There is also a marked difference between the solubility of phosphine in didecyl phthalate calculated from data given by Devyatykh and that in dibutyl phthalate measured by Palmer. Expressed as V01'PH3/vOl'solvent at 295.7 K the former gives 22.2 and the latter 3.23.