Chemical Weapons Technology Section 4—Chemical Weapons Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

Industry Compliance Programme
Global Chemical Industry Compliance Programme GC-ICP Chemical Weapons Convention December 2006 Version 1.0 GLOBAL CHEMICAL INDUSTRY COMPLIANCE PROGRAMME FOR IMPLEMENTING THE CHEMICAL WEAPONS CONVENTION The purpose of the handbook is to provide guidance to chemical facilities, traders and trading companies in developing a Global Chemical Industry Compliance Programme (GC-ICP) to comply with the Chemical Weapons Convention (CWC). The GC-ICP focuses first on determining if there is a reporting requirement to your National Authority and second on collecting the relevant support data used to complete the required reports. The GC-ICP is designed to provide a methodology to comply with the CWC and establish systems that facilitate and demonstrate such compliance. Each facility/company should also ensure that it follows its country’s CWC specific laws, regulations and reporting requirements. • Sections 2, 3, and 4 guide you through the process of determining if chemicals at your facility/ company should be reported to your National Authority for compliance with the CWC. • Section 5 provides recommended guidance on information that you may use to determine your reporting requirements under the CWC and administrative tools that your facility/company may use to ensure compliance with the CWC. • Section 6 provides a glossary of terms and associated acronyms. • Section 7 provides a listing of all National Authorities by country. CWC Global Chemical Industry Compliance Programme 1 TABLE OF CONTENTS Section 1 Overview What is the Chemical Weapons Convention? -

A Quantum Chemical Study Involving Nitrogen Mustards
The Pharmaceutical and Chemical Journal, 2016, 3(4):58-60 Available online www.tpcj.org ISSN: 2349-7092 Research Article CODEN(USA): PCJHBA Formation enthalpy and number of conformers as suitable QSAR descriptors: a quantum chemical study involving nitrogen mustards Robson Fernandes de Farias Universidade Federal do Rio Grande do Norte, Cx. Postal 1664, 59078-970, Natal-RN, Brasil Abstract In the present work, a quantum chemical study (Semi-empirical,PM6 method) is performed using nitrogen mustards (HN1, HN2 and HN3) as subjects in order to demonstrate that there is a close relationship between pharmacological activity and parameters such as formation enthalpy and number of conformers, which could, consequently, be employed as reliable QSAR descriptors. To the studied nitrogen mustards, a very simple equation o o relating log P, ΔH f and the number of conformers (Nc) was found: log P = [(log -ΔH f + logNc)/2]-0.28. Keywords QSAR, Descriptors, Formation enthalpy, Conformers, Semi-empirical, Nitrogen mustards, Log P Introduction It is well known that lipophilicity is a very important molecular descriptor that often correlates well with the bioactivity of chemicals [1]. Hence, lipophilicity, measured as log P, is a key property in quantitative structure activity relationship (QSAR) studies. In this connection, in the pharmaceutical sciences it is a common practice to use log P (the partition coefficient between water and octanol), as a reliable indicator of the hydrophobicity or lipophilicity of (drug) molecules [1-2]. For example, relying primarily on the log P is a sensible strategy in preparing future 18-crown-6 analogs with optimized biological activity [3]. -

Chemical Warfare Agent (CWA) Identification Overview
Physicians for Human Rights Chemical Warfare Agent (CWA) Identification Overview Chemical Warfare Agent Identification Fact Sheet Series Table of Contents This Chemical Warfare Agent (CWA) Identification Fact Sheet is part 2 Physical Properties of a Physicians for Human Rights (PHR) series designed to fill a gap in 2 VX (Nerve Agent) 2 Sarin (Nerve Agent) knowledge among medical first responders to possible CWA attacks. 2 Tabun (Nerve Agent) This document in particular outlines differences between a select 2 BZ (Incapacitating Agent) group of vesicants and nerve agents, the deployment of which would 2 Mustard Gas (Vesicant) necessitate emergency medical treatment and documentation. 3 Collecting Samples to Test for Exposure 4 Protection PHR hopes that, by referencing these fact sheets, medical professionals 5 Symptoms may be able to correctly diagnose, treat, and document evidence of 6 Differential Diagnosis exposure to CWAs. Information in this fact sheet has been compiled from 8 Decontimanation 9 Treatment publicly available sources. 9 Abbreviations A series of detailed CWA fact sheets outlining in detail those properties and treatment regimes unique to each CWA is available at physiciansforhumanrights.org/training/chemical-weapons. phr.org Chemical Warfare Agent (CWA) Identification Overview 1 Collect urine samples, and blood and hair samples if possible, immediately after exposure Physical Properties VX • A lethal dose (10 mg) of VX, absorbed through the skin, can kill within minutes (Nerve Agent) • Can remain in environment for weeks -

Medicine with Deeper, More Biochemical Conception Of
Edinburgh Medical Journal May 1951 APPLICATIONS of chemical defence research IN MEDICINET? By Professor R. A. PETERS to be invited It is a great responsibility, as well as a great honour, to Cameron lecturers give this lecture ; and I expect that with other I this ancient seat of share a sense of awe upon standing here in is learning. For me it is an awe tinged with much feeling (it possible that this is partly because some forebears of my mother came from near to recent of the this ; it is in part due the perusal city) perhaps " address here Froude in on the Times of John Knox, given by 1865 " " entitled Influence of the Reformation upon the Scottish Character ; this address drives home to the Sassenach the sterner stuff of which all of us in the the Scots are made ; but I really think it is more that as south, even if we come as I do from a Medical School as ancient the Hospital of St Bartholomew, know full well that Edinburgh stands high indeed in the world of Medical Schools as Scotland itself stands in the world of intellect. Elsewhere I have discussed fully the historical background of our for me back researches upon Chemical Defence substances, which go to World War I.23 Again in my Dixon lecture,24 I have indicated some of the interest for pharmacology. The outlook for medicine has not the been reviewed so far. Therefore I propose to-day to discuss of British influence upon the future of therapeutics of the discovery anti-lewisite and of some of the other researches upon war gas but substances ; and to consider not only the leads towards therapy, the possible newer trends given to medicine itself. -

Chlorine.Pdf
Chlorine 7782-50-5 Hazard Summary Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. Chronic (long-term) exposure to chlorine gas in workers has resulted in respiratory effects, including eye and throat irritation and airflow obstruction. No information is available on the carcinogenic effects of chlorine in humans from inhalation exposure. A National Toxicology Program (NTP) study showed no evidence of carcinogenic activity in male rats or male and female mice, and equivocal evidence in female rats, from ingestion of chlorinated water. EPA has not classified chlorine for potential carcinogenicity. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (2), which contains information on oral chronic toxicity and the RfD, The California Environmental Protection Agency's (CalEPA's) Technical Support Document for the Determination of Noncancer Chronic Reference Exposure Levels (3), and EPA's Drinking Water Criteria Document for Chlorine, Hypochlorous Acid and Hypochlorite Ion (1). Uses Chlorine is a commonly used household cleaner and disinfectant. It is widely used as an oxidizing agent in water treatment and chemical processes. It is also used in the bleaching process of wood pulp in pulp mills. (8) Sources and Potential Exposure Workers may be exposed to chlorine in industries where it is produced or used, particularly in the food and paper industries. In addition, persons breathing air around these industries may be exposed to chlorine. (1) Exposure to chlorine may also occur through drinking water and swimming pool water, where it is used as a disinfectant. -
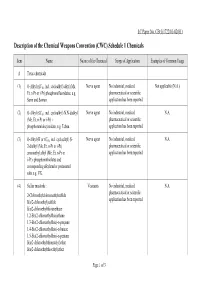
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Monoethanolamine Diethanolamine Triethanolamine DSA9781.Qxd 1/31/03 10:21 AM Page 2
DSA9781.qxd 1/31/03 10:21 AM Page 1 ETHANOLAMINES Monoethanolamine Diethanolamine Triethanolamine DSA9781.qxd 1/31/03 10:21 AM Page 2 CONTENTS Introduction ...............................................................................................................................2 Ethanolamine Applications.........................................................................................................3 Gas Sweetening ..................................................................................................................3 Detergents, Specialty Cleaners, Personal Care Products.......................................................4 Textiles.................................................................................................................................4 Metalworking ......................................................................................................................5 Other Applications...............................................................................................................5 Ethanolamine Physical Properties ...............................................................................................6 Typical Physical Properties ....................................................................................................6 Vapor Pressure of Ethanolamines (Figure 1).........................................................................7 Heat of Vaporization of Ethanolamines (Figure 2)................................................................7 Specific -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

Decision-Making in Chemical Warfare Agent (CWA) Response There Is a Lot of Fear Associated with Chemical Will Act Accordingly
Application Note: 102 Decision-Making in Chemical Warfare Agent (CWA) Response There is a lot of fear associated with Chemical will act accordingly. If the first responders Warfare Agents (CWAs). The misnomer over-react and immediately jump into full “Nerve Gas” quickly brings horrible images to encapsulation protection it could panic the the minds of many civilians. But if we lay aside public and cause unnecessary worry and the politics and fear, CWA detection should even injury. treated like other gas/vapor detection challenges. It should be a collaborative Over Protection Can Be Dangerous process encompassing physical clues, threat to the Responder scenario, biological clues, and a variety of Heat stress is the number one injury in sensing technologies. No one clue or HazMat response and immediately jumping technology is always correct. Experience and into full Level A encapsulation is a good way the use of multiple clues and technologies are of overheating oneself. Level A the keys to successful CWA response. encapsulation also makes one much more Understanding what the clues are and how to susceptible to slip, trip and fall injuries. layer them to make a decision is critical to Finally, over protection makes it harder to get successful CWA response. things done. When properly used, detection allows responders to respond at lower levels Why is Gas Detection Important? of Personal Protective Equipment (PPE) to Responders cannot rely on their senses for provide the highest levels of safety to decision-making. Without effectively knowing themselves and to the community that they how to use detection techniques responders protect. -

Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas
processes Article Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas Usman Shoukat , Diego D. D. Pinto and Hanna K. Knuutila * Department of Chemical Engineering, Norwegian University of Science and Technology (NTNU), 7491 Trondheim, Norway; [email protected] (U.S.); [email protected] (D.D.D.P.) * Correspondence: [email protected] Received: 8 February 2019; Accepted: 8 March 2019; Published: 15 March 2019 Abstract: Various novel amine solutions both in aqueous and non-aqueous [monoethylene glycol (MEG)/triethylene glycol(TEG)] forms have been studied for hydrogen sulfide (H2S) absorption. The study was conducted in a custom build experimental setup at temperatures relevant to subsea operation conditions and atmospheric pressure. Liquid phase absorbed H2S, and amine concentrations were measured analytically to calculate H2S loading (mole of H2S/mole of amine). Maximum achieved H2S loadings as the function of pKa, gas partial pressure, temperature and amine concentration are presented. Effects of solvent type on absorbed H2S have also been discussed. Several new solvents showed higher H2S loading as compared to aqueous N-Methyldiethanolamine (MDEA) solution which is the current industrial benchmark compound for selective H2S removal in natural gas sweetening process. Keywords: H2S absorption; amine solutions; glycols; desulfurization; aqueous and non-aqueous solutions 1. Introduction Natural gas is considered one of the cleanest forms of fossil fuel. Its usage in industrial processes and human activities is increasing worldwide, providing 23.4% of total world energy requirement in 2017 [1]. Natural gas is half of the price of crude oil and produces 29% less carbon dioxide than oil per unit of energy output [2]. -

Warning: the Following Lecture Contains Graphic Images
What the новичок (Novichok)? Why Chemical Warfare Agents Are More Relevant Than Ever Matt Sztajnkrycer, MD PHD Professor of Emergency Medicine, Mayo Clinic Medical Toxicologist, Minnesota Poison Control System Medical Director, RFD Chemical Assessment Team @NoobieMatt #ITLS2018 Disclosures In accordance with the Accreditation Council for Continuing Medical Education (ACCME) Standards, the American Nurses Credentialing Center’s Commission (ANCC) and the Commission on Accreditation for Pre-Hospital Continuing Education (CAPCE), states presenters must disclose the existence of significant financial interests in or relationships with manufacturers or commercial products that may have a direct interest in the subject matter of the presentation, and relationships with the commercial supporter of this CME activity. The presenter does not consider that it will influence their presentation. Dr. Sztajnkrycer does not have a significant financial relationship to report. Dr. Sztajnkrycer is on the Editorial Board of International Trauma Life Support. Specific CW Agents Classes of Chemical Agents: The Big 5 The “A” List Pulmonary Agents Phosgene Oxime, Chlorine Vesicants Mustard, Phosgene Blood Agents CN Nerve Agents G, V, Novel, T Incapacitating Agents Thinking Outside the Box - An Abbreviated List Ammonia Fluorine Chlorine Acrylonitrile Hydrogen Sulfide Phosphine Methyl Isocyanate Dibotane Hydrogen Selenide Allyl Alcohol Sulfur Dioxide TDI Acrolein Nitric Acid Arsine Hydrazine Compound 1080/1081 Nitrogen Dioxide Tetramine (TETS) Ethylene Oxide Chlorine Leaks Phosphine Chlorine Common Toxic Industrial Chemical (“TIC”). Why use it in war/terror? Chlorine Density of 3.21 g/L. Heavier than air (1.28 g/L) sinks. Concentrates in low-lying areas. Like basements and underground bunkers. Reacts with water: Hypochlorous acid (HClO) Hydrochloric acid (HCl).