Synthesis of Nerve Agent- and Pesticide-Protein Bioadducts As Reference Materials for Retrospective Verification of Exposure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -
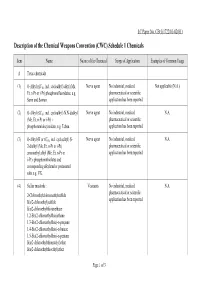
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

Lifetime Organophosphorous Insecticide Use Among Private Pesticide Applicators in the Agricultural Health Study
Journal of Exposure Science and Environmental Epidemiology (2012) 22, 584 -- 592 & 2012 Nature America, Inc. All rights reserved 1559-0631/12 www.nature.com/jes ORIGINAL ARTICLE Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study Jane A. Hoppin1, Stuart Long2, David M. Umbach3, Jay H. Lubin4, Sarah E. Starks5, Fred Gerr5, Kent Thomas6, Cynthia J. Hines7, Scott Weichenthal8, Freya Kamel1, Stella Koutros9, Michael Alavanja9, Laura E. Beane Freeman9 and Dale P. Sandler1 Organophosphorous insecticides (OPs) are the most commonly used insecticides in US agriculture, but little information is available regarding specific OP use by individual farmers. We describe OP use for licensed private pesticide applicators from Iowa and North Carolina in the Agricultural Health Study (AHS) using lifetime pesticide use data from 701 randomly selected male participants collected at three time periods. Of 27 OPs studied, 20 were used by 41%. Overall, 95% had ever applied at least one OP. The median number of different OPs used was 4 (maximum ¼ 13). Malathion was the most commonly used OP (74%) followed by chlorpyrifos (54%). OP use declined over time. At the first interview (1993--1997), 68% of participants had applied OPs in the past year; by the last interview (2005--2007), only 42% had. Similarly, median annual application days of OPs declined from 13.5 to 6 days. Although OP use was common, the specific OPs used varied by state, time period, and individual. Much of the variability in OP use was associated with the choice of OP, rather than the frequency or duration of application. -

The List of Extremely Hazardous Substances)
APPENDIX A (THE LIST OF EXTREMELY HAZARDOUS SUBSTANCES) THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 75-86-5 ACETONE CYANOHYDRIN 500 10 1752-30-3 ACETONE THIOSEMICARBAZIDE 500/500 1,000 107-02-8 ACROLEIN 500 1 79-06-1 ACRYLAMIDE 500/500 5,000 107-13-1 ACRYLONITRILE 500 100 814-68-6 ACRYLYL CHLORIDE 100 100 111-69-3 ADIPONITRILE 500 1,000 116-06-3 ALDICARB 100/500 1 309-00-2 ALDRIN 500/500 1 107-18-6 ALLYL ALCOHOL 500 100 107-11-9 ALLYLAMINE 500 500 20859-73-8 ALUMINUM PHOSPHIDE 500 100 54-62-6 AMINOPTERIN 500/500 500 78-53-5 AMITON 500 500 3734-97-2 AMITON OXALATE 100/500 100 7664-41-7 AMMONIA 500 100 300-62-9 AMPHETAMINE 500 1,000 62-53-3 ANILINE 500 5,000 88-05-1 ANILINE,2,4,6-TRIMETHYL- 500 500 7783-70-2 ANTIMONY PENTAFLUORIDE 500 500 1397-94-0 ANTIMYCIN A 500/500 1,000 86-88-4 ANTU 500/500 100 1303-28-2 ARSENIC PENTOXIDE 100/500 1 THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 1327-53-3 ARSENOUS OXIDE 100/500 1 7784-34-1 ARSENOUS TRICHLORIDE 500 1 7784-42-1 ARSINE 100 100 2642-71-9 AZINPHOS-ETHYL 100/500 100 86-50-0 AZINPHOS-METHYL 10/500 1 98-87-3 BENZAL CHLORIDE 500 5,000 98-16-8 BENZENAMINE, 3-(TRIFLUOROMETHYL)- 500 500 100-14-1 BENZENE, 1-(CHLOROMETHYL)-4-NITRO- 500/500 500 98-05-5 BENZENEARSONIC ACID 10/500 10 3615-21-2 BENZIMIDAZOLE, 4,5-DICHLORO-2-(TRI- 500/500 500 FLUOROMETHYL)- 98-07-7 BENZOTRICHLORIDE 100 10 100-44-7 BENZYL CHLORIDE 500 100 140-29-4 BENZYL CYANIDE 500 500 15271-41-7 BICYCLO[2.2.1]HEPTANE-2-CARBONITRILE,5- -

744 Hydrolysis of Chiral Organophosphorus Compounds By
[Frontiers in Bioscience, Landmark, 26, 744-770, Jan 1, 2021] Hydrolysis of chiral organophosphorus compounds by phosphotriesterases and mammalian paraoxonase-1 Antonio Monroy-Noyola1, Damianys Almenares-Lopez2, Eugenio Vilanova Gisbert3 1Laboratorio de Neuroproteccion, Facultad de Farmacia, Universidad Autonoma del Estado de Morelos, Morelos, Mexico, 2Division de Ciencias Basicas e Ingenierias, Universidad Popular de la Chontalpa, H. Cardenas, Tabasco, Mexico, 3Instituto de Bioingenieria, Universidad Miguel Hernandez, Elche, Alicante, Spain TABLE OF CONTENTS 1. Abstract 2. Introduction 2.1. Organophosphorus compounds (OPs) and their toxicity 2.2. Metabolism and treatment of OP intoxication 2.3. Chiral OPs 3. Stereoselective hydrolysis 3.1. Stereoselective hydrolysis determines the toxicity of chiral compounds 3.2. Hydrolysis of nerve agents by PTEs 3.2.1. Hydrolysis of V-type agents 3.3. PON1, a protein restricted in its ability to hydrolyze chiral OPs 3.4. Toxicity and stereoselective hydrolysis of OPs in animal tissues 3.4.1. The calcium-dependent stereoselective activity of OPs associated with PON1 3.4.2. Stereoselective hydrolysis commercial OPs pesticides by alloforms of PON1 Q192R 3.4.3. PON1, an enzyme that stereoselectively hydrolyzes OP nerve agents 3.4.4. PON1 recombinants and stereoselective hydrolysis of OP nerve agents 3.5. The activity of PTEs in birds 4. Conclusions 5. Acknowledgments 6. References 1. ABSTRACT Some organophosphorus compounds interaction of the racemic OPs with these B- (OPs), which are used in the manufacturing of esterases (AChE and NTE) and such interactions insecticides and nerve agents, are racemic mixtures have been studied in vivo, ex vivo and in vitro, using with at least one chiral center with a phosphorus stereoselective hydrolysis by A-esterases or atom. -

Pacs by Chemical Name (Mg/M3) (Pdf)
Table 4: Protective Action Criteria (PAC) Rev 25 based on applicable 60-minute AEGLs, ERPGs, or TEELs. Values are presented in mg/m3. August 2009 Table 4 is an alphabetical listing of the chemicals in the PAC data set. It provides Chemical Abstract Service Registry Numbers (CASRNs)1, PAC values, and technical information on the source of the PAC values. Table 4 presents all values for TEEL-0, PAC-1, PAC-2, and PAC-3 in mg/m3. The conversion of ppm to mg/m3 is calculated assuming 25 ºC and 760 mm Hg. The columns presented in Table 4 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this alphabetical listing. Chemical Name The chemical name given to the PAC Development Team. CASRN The Chemical Abstract Service Registry Number for this chemical. TEEL-0 This is the threshold concentration below which most people will experience no adverse health effects. This PAC is always based on TEEL-0 because AEGL-0 or ERPG-0 values do not exist. PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value. PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value. PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value. Source of PACs: Technical comments provided by the PAC development team that TEEL-0, PAC-1, indicate the source of the data used to derive PAC values. Future efforts PAC-2, PAC-3 are directed at reviewing, revising, and enhancing this information. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Soil Degradation Comparisons of Corn Rootworm Insecticides in the Field and Laboratory
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Electronic Theses and Dissertations 1987 Soil Degradation Comparisons of Corn Rootworm Insecticides in the Field and Laboratory Michael J. Haas Follow this and additional works at: https://openprairie.sdstate.edu/etd Recommended Citation Haas, Michael J., "Soil Degradation Comparisons of Corn Rootworm Insecticides in the Field and Laboratory" (1987). Electronic Theses and Dissertations. 4447. https://openprairie.sdstate.edu/etd/4447 This Thesis - Open Access is brought to you for free and open access by Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. SOIL DEGRADATION COMPARISONS OF CORN ROOTWORM INSECTICIDES IN THE FIELD AND LABORATORY BY MICHAEL J . HAAS A thesis submitted in partial fulfillment of the requirements for the degree Master of Science Major in Entomo logy South Dakota State Univers ity 1987 SOIL DEGRADATION COMPARISONS OF INSECTICIDES IN THE FIELD AND LA BORATORY This thesis is approved as a creditable and independent investigation by a cand idate for the degree , Master of Science , and is acceptable for meeting the thesis requirements for this degree . Acceptance of this thesis does not imply that the conclusions reached by the candidate are necessarily the conclusions of the major department . David D. Walgenbach Thesis Adviser j MaJrlce L. Horton Date �ead , Dept . of Plant Science ACKNOWLEDGEMENTS I would like to extend my sincere gratitude to Dr . -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

459 Part 121—The United States Munitions List
Department of State § 121.1 (h) Equipment is a combination of 121.1 The United States Munitions List. parts, components, accessories, attach- 121.2–121.15 [Reserved] ments, firmware, or software that oper- 121.16 Missile Technology Control Regime ate together to perform a function of, Annex. as, or for an end-item or system. AUTHORITY: Secs. 2, 38, and 71, Pub. L. 90– Equipment may be a subset of an end- 629, 90 Stat. 744 (22 U.S.C. 2752, 2778, 2797); 22 item based on the characteristics of U.S.C. 2651a; Pub. L. 105–261, 112 Stat. 1920; Section 1261, Pub. L. 112–239; E.O. 13637, 78 FR the equipment. Equipment that meets 16129. the definition of an end-item is an end- item. Equipment that does not meet SOURCE: 58 FR 39287, July 22, 1993, unless the definition of an end-item is a com- otherwise noted. ponent, accessory, attachment, ENUMERATION OF ARTICLES firmware, or software. [79 FR 61228, Oct. 10, 2014] § 121.1 The United States Munitions List. § 120.46 Classified. (a) U.S. Munitions List. In this part, Classified means classified pursuant articles, services, and related technical to Executive Order 13526, and a secu- data are designated as defense articles rity classification guide developed pur- or defense services pursuant to sections suant thereto or equivalent, or to the 38 and 47(7) of the Arms Export Control corresponding classification rules of Act and constitute the U.S. Munitions another government or international List (USML). Changes in designations organization. are published in the FEDERAL REG- ISTER. -

ITAR Category
Category XIV—Toxicological Agents, Including Chemical Agents, Biological Agents, and Associated Equipment *(a) Chemical agents, to include: (1) Nerve agents: (i) O-Alkyl (equal to or less than C10, including cycloalkyl) alkyl (Methyl, Ethyl, n-Propyl or Isopropyl)phosphonofluoridates, such as: Sarin (GB): O-Isopropyl methylphosphonofluoridate (CAS 107–44–8) (CWC Schedule 1A); and Soman (GD): O-Pinacolyl methylphosphonofluoridate (CAS 96–64–0) (CWC Schedule 1A); (ii) O-Alkyl (equal to or less than C10, including cycloalkyl) N,N-dialkyl (Methyl, Ethyl, n- Propyl or Isopropyl)phosphoramidocyanidates, such as: Tabun (GA): O-Ethyl N, N- dimethylphosphoramidocyanidate (CAS 77–81–6) (CWC Schedule 1A); (iii) O-Alkyl (H or equal to or less than C10, including cycloalkyl) S–2-dialkyl (Methyl, Ethyl, n- Propyl or Isopropyl)aminoethyl alkyl (Methyl, Ethyl, n-Propyl or Isopropyl)phosphonothiolates and corresponding alkylated and protonated salts, such as: VX: O-Ethyl S-2- diisopropylaminoethyl methyl phosphonothiolate (CAS 50782–69–9) (CWC Schedule 1A); (2) Amiton: O,O-Diethyl S-[2(diethylamino)ethyl] phosphorothiolate and corresponding alkylated or protonated salts (CAS 78–53–5) (CWC Schedule 2A); (3) Vesicant agents: (i) Sulfur mustards, such as: 2-Chloroethylchloromethylsulfide (CAS 2625–76–5) (CWC Schedule 1A); Bis(2-chloroethyl)sulfide (CAS 505–60–2) (CWC Schedule 1A); Bis(2- chloroethylthio)methane (CAS 63839–13–6) (CWC Schedule 1A); 1,2-bis (2- chloroethylthio)ethane (CAS 3563–36–8) (CWC Schedule 1A); 1,3-bis (2-chloroethylthio)-n- propane (CAS