Bacterial Infections and Infectious Dermatologic Emergencies.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Smelly Foot Rash
CLINICAL Smelly foot rash Paulo Morais Ligia Peralta Keywords: skin diseases, infectious Case study A previously healthy Caucasian girl, 6 years of age, presented with pruritic rash on both heels of 6 months duration. The lesions appeared as multiple depressions 1–2 mm in diameter that progressively increased in size. There was no history of trauma or insect bite. She reported local pain when walking, worse with moisture and wearing sneakers. On examination, multiple small crater- like depressions were present, some Figure 1. Heel of patient coalescing into a larger lesion on both heels (Figure 1). There was an unpleasant ‘cheesy’ protective/occluded footwear for prolonged odour and a moist appearance. Wood lamp periods.1–4 examination and potassium hydroxide testing for fungal hyphae were negative. Answer 2 Question 1 Pitted keratolysis is frequently seen during What is the diagnosis? summer and rainy seasons, particularly in tropical regions, although it occurs Question 2 worldwide.1,3,4 It is caused by Kytococcus What causes this condition? sedentarius, Dermatophilus congolensis, or species of Corynebacterium, Actinomyces or Question 3 Streptomyces.1–4 Under favourable conditions How would you confirm the diagnosis? (ie. hyperhidrosis, prolonged occlusion and increased skin surface pH), these bacteria Question 4 proliferate and produce proteinases that destroy What are the differential diagnoses? the stratum corneum, creating pits. Sulphur containing compounds produced by the bacteria Question 5 cause the characteristic malodor. What is your management strategy? Answer 3 Answer 1 Pitted keratolysis is usually a clinical Based on the typical clinical picture and the negative diagnosis with typical hyperhidrosis, malodor ancillary tests, the diagnosis of pitted keratolysis (PK) (bromhidrosis) and occasionally, tenderness, is likely. -

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

Bacterial Skin Infections
BACTERIAL SKIN INFECTIONS SPEAKER: DR LUIZ ALBERTO BOMJARDIM PÔRTO DERMATOLOGIST BRAZIL MRSA INFECTIONS • Concept: Methicillin- resistant Staphylococcus aureus • Epidemiology: Gradual increase of resistance. • Nosocomial MRSA risk factors: Hospitalization, ICU, invasive procedures, previous antibiotic therapy, health professionals, diabetes mellitus, EV drugs, immunosuppression and chronic diseases. MRSA INFECTIONS • Community MARSA risk factors: Children, EV drugs, indigenous, homosexual men, military, prisoners and athletes. • Microorganisms more virulent by genetic characteristics. MRSA INFECTIONS • Clinic caracteristics: -Abscess, cellulitis, folliculitis, impetigo, infected wounds, external otitis, paronychia and colonization of the skin in cases of atopic dermatitis. - Increased morbidity. • Propedeutics: Culture blood, tissue or secretion. MRSA INFECTIONS • Treatment: - Pathology-specific treatment. - Prefer non-beta-lactam antibiotics, such as: clindamycin, sulfamethoxazole- trimethoprim and tetracyclines. - On suspicion of MARSA infection, start empirical antibiotics and stagger specific antibiotics by culture with antibiograma. MRSA INFECTIONS • Treatment: - Decolonization: systemic antibiotic therapy, topical 2% mupirocin, personal hygiene with antiseptic or antimicrobial solutions (iodine-povidine, chlorhexidine or triclosan). MRSA INFECTIONS • Prevention: - Avoid skin-to-skin contact and share personal belongings / clothing. - Hand washing. - Use of alcohol gels. - Cover wounds. - Isolation contact of MARSA carriers. - Early -

Approach to the Patient with Presumed Cellulitis Daniela Kroshinsky, MD,* Marc E
Approach to the Patient With Presumed Cellulitis Daniela Kroshinsky, MD,* Marc E. Grossman, MD, FACP,† and Lindy P. Fox, MD‡ Dermatologists frequently are consulted in the evaluation and management of the patient with cellulitic-appearing skin. For routine cellulitis, the clinical presentation and patient symptoms are usually sufficient for an accurate diagnosis. However, when the clinical presentation is somewhat atypical, or if the patient fails to respond to appropriate therapy for cellulitis because of routine bacterial pathogens, the differential diagnosis should be rapidly expanded. We discuss the approach to the patient with presumed cellulitis, with an emphasis on the differential diagnosis of cellulitis in both the immunocompetent and immunucompromised patient. Semin Cutan Med Surg 26:168-178 © 2007 Elsevier Inc. All rights reserved. KEYWORDS cellulitis, erysipelas 53-year-old woman with a history of recurrent breast of cellulitis, and telangiectasia and scattered enlarged mes- Acancer diagnosed 2 years before presentation and treated enchymal cells, characteristic of radiation changes. with radiation and chemotherapy (docetaxel, anastrozole, exemestane, gemcitabine) most recently 6 months before presentation was admitted for 3 weeks of worsening chest Clinical Problem wall pain and a rash over her mastectomy scar. Despite 5 days Dermatologists frequently are consulted in the evaluation of empiric antibiotic therapy with doxycycline and vancomy- and management of the patient with cellulitic-appearing cin, the chest wall erythema and pain were increasing. A dermatology consultation was called. An ulceration and sur- skin. Although the dermatologist may be consulted early on rounding erythematous papules were concentrated over the in the patient’s course, more often a dermatology consult is mastectomy scar with ill-defined erythematous patches that requested when a patient fails to respond to treatment. -
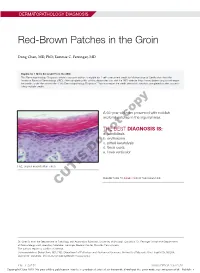
Red-Brown Patches in the Groin
DERMATOPATHOLOGY DIAGNOSIS Red-Brown Patches in the Groin Dong Chen, MD, PhD; Tammie C. Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 66-year-old man presented with reddish arciform patchescopy in the inguinal area. THE BEST DIAGNOSIS IS: a. candidiasis b. noterythrasma c. pitted keratolysis d. tinea cruris Doe. tinea versicolor H&E, original magnification ×600. PLEASE TURN TO PAGE 419 FOR THE DIAGNOSIS CUTIS Dr. Chen is from the Department of Pathology and Anatomical Sciences, University of Missouri, Columbia. Dr. Ferringer is from the Departments of Dermatology and Laboratory Medicine, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Dong Chen, MD, PhD, Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Dr, MA204, DC018.00, Columbia, MO 65212 ([email protected]). 416 I CUTIS® WWW.MDEDGE.COM/CUTIS Copyright Cutis 2018. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Erythrasma rythrasma usually involves intertriginous areas surface (Figure 1) compared to dermatophyte hyphae that (eg, axillae, groin, inframammary area). Patients tend to be parallel to the surface.2 E present with well-demarcated, minimally scaly, red- Pitted keratolysis is a superficial bacterial infection brown patches. -

Download Erythasma
ERYTHASMA What is it? Erythasma is a common skin condition that affects the following areas – the groin, under the arms and between the toes What does it look like? Erythrasma appears as well-defined scaly red, pink or brown patches. The most commonly affected areas are the groin, armpits, between the toes and in other skin folds. It can sometimes become generalized and affect larger areas on the body. The patches can sometimes be itchy or have an odor. What causes it? Erythrasma is an infection of the skin caused by an overgrowth of a bacterium called Corynebacterium minutissimum. It can affect healthy adults, but it more frequently affects older people with obesity, excessive sweating (hyperhidrosis [link]), diabetes mellitus or people who live in tropical climates. Infection is facilitated under conditions of moisture and occlusion. How is it diagnosed? Erythrasma is diagnosed based on the history and physical appearance of the lesions. A special lamp called a Wood’s lamp can be used to help confirm the diagnosis, as erythrasma glows bright pink under the lamp. How is it treated? Erythrasma can be treated with either topical (applied directly to the skin) or oral therapies. For patients with localised disease, a topical antibiotic such as clindamycin or erythromycin can be used. On the hand, it can be difficult to treat extensive areas with creams and so oral antibiotics (clindamycin or erythromycin) may be preferred. It is important to note that recurrence is common. What can be done to prevent it? In order to avoid recurrences, it is important to keep the skin as dry as possible and optimise pre-disposing conditions such as keeping diabetes well-controlled. -

Erythrasma Capitis and Diffuse Hair Loss with Patches and Eczema-A Rare but Underdiagnozed Entity? Rauno J
Annals of Case Reports Harvima RJ and Harvima IT Ann Case Report 6: 666. Case Report DOI: 10.29011/2574-7754.100666 Erythrasma Capitis and Diffuse Hair Loss with Patches and Eczema-a Rare but Underdiagnozed Entity? Rauno J. Harvima1*, Ilkka T. Harvima2 1Department of Dermatology, University of Eastern Finland, Kuopio University Hospital and Helsinki University Central Hospital, Finland 2Department of Dermatology, University of Eastern Finland and Kuopio University Hospital, Finland *Corresponding author: Rauno J. Harvima, Department of Dermatology, Kuopio University Hospital, Puijonlaaksontie 2, FIN- 70210 Kuopio, Finland Citation: Harvima RJ, Harvima IT. (2021) Erythrasma Capitis and Diffuse Hair Loss with Patches and Eczema-a Rare but Underdiagnozed Entity. Ann Case Report 6: 666. DOI: 10.29011/2574-7754.100666 Received Date: 11 May, 2021; Accepted Date: 14 May, 2021; Published Date: 17 May, 2021 Introduction Corynebacterium minutissimum is considered to belong to the normal flora of skin [1]. However, it can cause a skin disease erythrasma, especially in axillar area. Certain corynebacterium species are associated with skin infections [2,3], and pitted keratolysis. There are only a few reports published for animals regarding the association of corynebacterium and alopecia in one Beagle dog [4], and in two horses [5,6]. However, there are no reports in the literature or in Textbooks of Dermatology on the association of corynebacterium with scalp hair loss in humans. Due to only a few Figure 1a: Patient 1, a healthy 16-year-old woman suffering from patients seen in clinical practice, this association is likely rare, and scalp dermatitis and diffuse hair loss for 2 months, back of head. -

Bacterial Skin Infections
MYTHS AND FACTS: BACTERIAL SKIN INFECTIONS A better understanding of these often-serious infections that are rising in incidence and becoming more resistant to antibiotics is the first step to improved treatment. Ronale Tucker Rhodes, MS 38 BIO SUPPLY TRENDS QUARTERLY | Summer 2016 DURING THE PAST several years, disturbing headlines about can result in methicillin-resistant Staphylococcus aureas flesh-eating bacteria have raised fear among the public, but few (MRSA), which can be a life-threatening infection because think they’ll actually be the next victim. That was certainly true certain antibiotics in the penicillin family cannot treat it. 8 of Cindy Martinez, who, in May 2015, somehow contracted one Streptococcal infection also can cause many types of infections, strain of the dangerous bacteria known to cause necrotizing but it more regularly causes impetigo, which results in a rash fasciitis. A former Marine and mother of two small children, several days after infection with small blisters that burst and Cindy survived but only after her feet and right hand were leave crusty, golden patches on the skin — occurring most amputated to halt the bacteria’s effects. 1 Necrotizing fasciitis is commonly on the face. 5 Both Staphylococcus and Streptococcus rare. According to the Centers for Disease Control and also commonly cause cellulitis, which can occur anywhere on Prevention (CDC), which tracks specific infections in the U.S. the body; however, the most common location is the lower leg. 9 through a special system called Active Bacterial Core surveillance Cellulitis is a painful infection of the deeper layers of the skin (ABCs), there are about 650 to 850 cases of necrotizing fasciitis that appears as an area of redness, warmth and swelling that caused, predominantly, by group A Streptococcal bacteria each gradually spreads. -

World Journal of Clinical Pediatrics
World Journal of W J C P Clinical Pediatrics Submit a Manuscript: http://www.f6publishing.com World J Clin Pediatr 2018 October 25; 7(4): 89-104 DOI: 10.5409/wjcp.v7.i4.89 ISSN 2219-2808 (online) REVIEW Perianal infectious dermatitis: An underdiagnosed, unremitting and stubborn condition Elena Daniela Serban Elena Daniela Serban, 2nd Department of Pediatrics, “Iuliu ficial inflammation of the perianal skin, which is of bac Hatieganu” University of Medicine and Pharmacy, Emergency terial origin (classically, group A betahemolytic strepto Hospital for Children, Cluj-Napoca 400177, Romania cocci). This narrative review aims to critically review and summarize the available scientific literature regarding ORCID number: Elena Daniela Serban (0000-0003-0906-1232). pediatric PID, being the first of its kind, to the best of Author contributions: Serban ED contributed to the paper’s the author’s knowledge. It also reports the first cases of conception and design, the data collection, extraction, analysis Romanian children with PID. Multiple databases were and evaluation, the interpretation of results, and the manuscript’s subjected to systematic literature search (from 1966 preparation, critical revision, editing and final submission. to April 30, 2018) to identify studies and case reports of children with PID. As such, this review provides up Conflict-of-interest statement: The author declares there are no dated information about essential aspects of PID (epi potential conflicts of interest relevant to this publication. demiology, etiology, pathogenesis, as well as clinical OpenAccess: This article is an open-access article which was features, required investigations and therapeutic options) selected by an in-house editor and fully peer-reviewed by external and of diagnostic pitfalls. -

Reading List 2012.Indd
General Reading iGAS Guidelines - Published January 2012 CLICK HERE Educational Interim UK guidelines for management of close community contacts of invasive group A streptococcal disease. Health Protection Agency, Workshops 2012 Group A Streptococcus Working Group. Communicable Disease and Public Health 2004; 7(4):354-361. CLICK HERE Keynote Presentation: Diagnosis and Complicated infections of skin and skin structures: when the infection is more than skin deep. DiNubile MJ, Lipsky, B. Journal of treatment Antimicrobial Chemotherapy, 2004, 53, Suppl. S2, ii37-ii50 of skin and soft CLICK HERE Practice guidelines for the diagnosis and management of skin and tissue infections soft tissue infections. Stevens DL et al. Clinical Infectious Disease 2005; 41:1373–1406 CLICK HERE Infections of skin and soft tissue: Outcomes of a classifi cation scheme. Eron J. Clinical Infectious Diseases 2000;31:287(A432). CLICK HERE Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY READING Antimicrobial Surveillance Program (United States and Canada, 2000). Rennie RP et al. Diagn Microbiol Infect Dis. 2003 Apr; 45(4):287-293. LIST CLICK HERE Comparison of community and health care associated methicillin resistant Staphylococcus aureus infection. Naimi TS, et al. JAMA 2003; 290: 2976-2984 CLICK HERE Methicillin resistant S. aureus infections amoung patients in the emergency department. Moran GJ et al. The New England Journal of Medicine 2006 CLICK HERE HPR 2011;5(7): News CLICK HERE Polyclonal multiply antiobiotic-resistant methicillin-resistant Staphylococcus aureus with Panton-Valentine leucocidin in England. JAC 2009; doi: 10.1093/jac/dkp386; CLICK HERE Eff ect of antibiotics on Staphylococcus aureus producing panton- valentine leukocidin. -

Infectious Diseases of the Philippines
INFECTIOUS DISEASES OF THE PHILIPPINES Stephen Berger, MD Infectious Diseases of the Philippines - 2013 edition Infectious Diseases of the Philippines - 2013 edition Stephen Berger, MD Copyright © 2013 by GIDEON Informatics, Inc. All rights reserved. Published by GIDEON Informatics, Inc, Los Angeles, California, USA. www.gideononline.com Cover design by GIDEON Informatics, Inc No part of this book may be reproduced or transmitted in any form or by any means without written permission from the publisher. Contact GIDEON Informatics at [email protected]. ISBN-13: 978-1-61755-582-4 ISBN-10: 1-61755-582-7 Visit http://www.gideononline.com/ebooks/ for the up to date list of GIDEON ebooks. DISCLAIMER: Publisher assumes no liability to patients with respect to the actions of physicians, health care facilities and other users, and is not responsible for any injury, death or damage resulting from the use, misuse or interpretation of information obtained through this book. Therapeutic options listed are limited to published studies and reviews. Therapy should not be undertaken without a thorough assessment of the indications, contraindications and side effects of any prospective drug or intervention. Furthermore, the data for the book are largely derived from incidence and prevalence statistics whose accuracy will vary widely for individual diseases and countries. Changes in endemicity, incidence, and drugs of choice may occur. The list of drugs, infectious diseases and even country names will vary with time. Scope of Content: Disease designations may reflect a specific pathogen (ie, Adenovirus infection), generic pathology (Pneumonia - bacterial) or etiologic grouping (Coltiviruses - Old world). Such classification reflects the clinical approach to disease allocation in the Infectious Diseases Module of the GIDEON web application.