Dissertation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(Mollusca, Gastropoda) of the Bulgarian Part of the Alibotush Mts
Malacologica Bohemoslovaca (2008), 7: 17–20 ISSN 1336-6939 Terrestrial gastropods (Mollusca, Gastropoda) of the Bulgarian part of the Alibotush Mts. IVAILO KANEV DEDOV Central Laboratory of General Ecology, 2 Gagarin Str., BG-1113 Sofia, Bulgaria, e-mail: [email protected] DEDOV I.K., 2008: Terrestrial gastropods (Mollusca, Gastropoda) of the Bulgarian part of the Alibotush Mts. – Malacologica Bohemoslovaca, 7: 17–20. Online serial at <http://mollusca.sav.sk> 20-Feb-2008. This work presents results of two years collecting efforts within the project “The role of the alpine karst area in Bulgaria as reservoir of species diversity”. It summarizes distribution data of 44 terrestrial gastropods from the Bulgarian part of Alibotush Mts. Twenty-seven species are newly recorded from the Alibotush Mts., 13 were con- firmed, while 4 species, previously known from the literature, were not found. In the gastropod fauna of Alibotush Mts. predominate species from Mediterranean zoogeographic complex. A large part of them is endemic species, and this demonstrates the high conservation value of large limestone areas in respect of terrestrial gastropods. Key words: terrestrial gastropods, distribution, Alibotush Mts., Bulgaria Introduction Locality 6: vill. Katuntsi, Izvorite hut, near hut, open The Alibotush Mts. (other popular names: Kitka, Gotseva ruderal terrain, under bark, 731 m a.s.l., coll. I. Dedov. Planina, Slavjanka) is one of the most interesting large Locality 7: vill. Katuntsi, tufa-gorge near village, 700 m limestone area in Bulgaria (Fig. 1). It occupies the part a.s.l., coll. I. Dedov, N. Simov. of the border region between Bulgaria and Greece with Locality 8: below Livade area, road between Goleshevo maximum elevation 2212 m (Gotsev peak). -

ED45E Rare and Scarce Species Hierarchy.Pdf
104 Species 55 Mollusc 8 Mollusc 334 Species 181 Mollusc 28 Mollusc 44 Species 23 Vascular Plant 14 Flowering Plant 45 Species 23 Vascular Plant 14 Flowering Plant 269 Species 149 Vascular Plant 84 Flowering Plant 13 Species 7 Mollusc 1 Mollusc 42 Species 21 Mollusc 2 Mollusc 43 Species 22 Mollusc 3 Mollusc 59 Species 30 Mollusc 4 Mollusc 59 Species 31 Mollusc 5 Mollusc 68 Species 36 Mollusc 6 Mollusc 81 Species 43 Mollusc 7 Mollusc 105 Species 56 Mollusc 9 Mollusc 117 Species 63 Mollusc 10 Mollusc 118 Species 64 Mollusc 11 Mollusc 119 Species 65 Mollusc 12 Mollusc 124 Species 68 Mollusc 13 Mollusc 125 Species 69 Mollusc 14 Mollusc 145 Species 81 Mollusc 15 Mollusc 150 Species 84 Mollusc 16 Mollusc 151 Species 85 Mollusc 17 Mollusc 152 Species 86 Mollusc 18 Mollusc 158 Species 90 Mollusc 19 Mollusc 184 Species 105 Mollusc 20 Mollusc 185 Species 106 Mollusc 21 Mollusc 186 Species 107 Mollusc 22 Mollusc 191 Species 110 Mollusc 23 Mollusc 245 Species 136 Mollusc 24 Mollusc 267 Species 148 Mollusc 25 Mollusc 270 Species 150 Mollusc 26 Mollusc 333 Species 180 Mollusc 27 Mollusc 347 Species 189 Mollusc 29 Mollusc 349 Species 191 Mollusc 30 Mollusc 365 Species 196 Mollusc 31 Mollusc 376 Species 203 Mollusc 32 Mollusc 377 Species 204 Mollusc 33 Mollusc 378 Species 205 Mollusc 34 Mollusc 379 Species 206 Mollusc 35 Mollusc 404 Species 221 Mollusc 36 Mollusc 414 Species 228 Mollusc 37 Mollusc 415 Species 229 Mollusc 38 Mollusc 416 Species 230 Mollusc 39 Mollusc 417 Species 231 Mollusc 40 Mollusc 418 Species 232 Mollusc 41 Mollusc 419 Species 233 -

Middle and Late Pleistocene Loess-Palaeosol Archives in East Croatia: Multi-Proxy Palaeoecological Studies on Zmajevac and Šarengrad Ii Sequences
Studia Quaternaria, vol. 38, no. 1 (2021): 3–17 DOI: 10.24425/sq.2020.133758 MIDDLE AND LATE PLEISTOCENE LOESS-PALAEOSOL ARCHIVES IN EAST CROATIA: MULTI-PROXY PALAEOECOLOGICAL STUDIES ON ZMAJEVAC AND ŠARENGRAD II SEQUENCES Dávid Molnár1, 2*, László Makó1, 2, Péter Cseh1, 2, Pál Sümegi1, 2, István Fekete3, Lidija Galović4 1 Department of Geology and Paleontology, University of Szeged, H-6722 Szeged, Egyetem u. 2-6, Hungary; e-mail: [email protected] 2 University of Szeged, Interdisciplinary Excellence Centre, Institute of Geography and Earth Sciences, Long Environmental Changes research team, H-6722 Szeged, Egyetem u. 2-6, Hungary; 3 Department of Physical Geography and Geoinformatics, University of Szeged, H-6722 Szeged, Egyetem u. 2-6, Hungary; 4 Croatian Geological Survey, Sachsova 2, 10001 Zagreb, Croatia. * corresponding author Abstract: Multi-proxy palaeoenvironmental analyses on the two loess-palaeosol sequences of Šarengrad II and Zmajevac (Croatia) provided the opportunity to obtain various data on climatic and environmental events that occurred in the southern part of the Carpathian Basin during the past 350,000 years. Palaeoecological horizons were reconstructed using sedimentological data (organic matter and carbonate content, grain-size distribution and magnetic susceptibility) and the dominance-based malacological results (MZs) supported by habitat and richness charts, moreover multi-variate statistics (cluster analysis). The correlation of the reconstructed palaeoecological horizons with global climatic trends (Marine Isotope Stages) determined the main accumulation processes in the examined areas. The palaeoecological analyses revealed specific accumulation conditions at both sequences, fluvial and aeolian environments at Šarengrad and a possible forest refuge at Zmajevac. Key words: palaeoecology, malacology, sedimentology, Šarengrad, Zmajevac, Croatia Manuscript received 17 March 2020, accepted 1 October 2020 INTRODUCTION palaeosol sequences (Zmajevac and Šarengrad II), situated on the right bank of the Danube River (Fig. -

Revision of the Systematic Position of Lindbergia Garganoensis
Revision of the systematic position of Lindbergia garganoensis Gittenberger & Eikenboom, 2006, with reassignment to Vitrea Fitzinger, 1833 (Gastropoda, Eupulmonata, Pristilomatidae) Gianbattista Nardi Via Boschette 8A, 25064 Gussago (Brescia), Italy; [email protected] [corresponding author] Antonio Braccia Via Ischia 19, 25100 Brescia, Italy; [email protected] Simone Cianfanelli Museum System of University of Florence, Zoological Section “La Specola”, Via Romana 17, 50125 Firenze, Italy; [email protected] & Marco Bodon c/o Museum System of University of Florence, Zoological Section “La Specola”, Via Romana 17, 50125 Firenze, Italy; [email protected] Nardi, G., Braccia, A., Cianfanelli, S. & Bo- INTRODUCTION don, M., 2019. Revision of the systematic position of Lindbergia garganoensis Gittenberger & Eiken- Lindbergia garganoensis Gittenberger & Eikenboom, 2006 boom, 2006, with reassignment to Vitrea Fitzinger, is the first species of the genus, Lindbergia Riedel, 1959 to 1833 (Gastropoda, Eupulmonata, Pristilomatidae). be discovered in Italy. The genus Lindbergia encompasses – Basteria 83 (1-3): 19-28. Leiden. Published 6 April 2019 about ten different species, endemic to the Greek mainland, Crete, the Cycladic islands, Dodecanese islands, northern Aegean islands, and southern Turkey (Riedel, 1992, 1995, 2000; Welter-Schultes, 2012; Bank & Neubert, 2017). Due to Lindbergia garganoensis Gittenberger & Eikenboom, 2006, lack of anatomical data, some of these species remain ge- a taxon with mainly a south-Balkan distribution, is the only nerically questionable. Up to now, L. garganoensis was only Italian species assigned to the genus Lindbergia Riedel, 1959. known by the presence of very fine spiral striae on the tel- The assignment to this genus, as documented by the pecu- eoconch and by the general shape of its shell. -

Compositional Variability of Pleistocene Land Snail Assemblages Preserved in a Cinder Cone
Compositional variability of Pleistocene land snail assemblages preserved in a cinder cone volcano from Tenerife, Canary Islands A thesis submitted to the graduate school of the University of Cincinnati in partial fulfillment of the requirements for the degree of Master of Science In the Department of Geology of the College of Arts and Sciences by Elizabeth M. Bullard B.S., Muskingum University, 2012 July 2016 Advisors: Dr. Yurena Yanes Dr. Arnold I. Miller Committee Member: Dr. Joshua Miller i Abstract Fossil assemblage faunal compositions may vary through space and time in response to climatic and/or taphonomic factors, but these relationships can be difficult to diagnose and disentangle. Here, we investigate how to disentangle climatic and taphonomic signals of a land- snail-rich volcanic scoria sequence to asses if it was influenced by taphonomic bias, climate change, or both, using a multifaceted approach, combining taphonomic, ecological, body-size, and stable-isotope data. Fossil assemblages were sampled from two beds (Units A and B) in a Pleistocene cinder cone volcano of southern Tenerife (Canary Islands), dated to the glacial interval MIS 8 (~299-302 ka). The two units differed in taphonomy, species composition, and abundance distributions. The upper unit, B (6 species), showed higher snail diversity and shell concentration and lower taphonomic alteration than the lower unit, A (3 species). Furthermore, larger bodied species (length>10mm) dominated Unit A and were better preserved than smaller species (length<10mm), whereas smaller individuals were more abundant (and better preserved) at Unit B. These differences were likely impacted by physical differences in the matrices surrounding the fossils. -

Malaco Le Journal Électronique De La Malacologie Continentale Française
MalaCo Le journal électronique de la malacologie continentale française www.journal-malaco.fr MalaCo (ISSN 1778-3941) est un journal électronique gratuit, annuel ou bisannuel pour la promotion et la connaissance des mollusques continentaux de la faune de France. Equipe éditoriale Jean-Michel BICHAIN / Paris / [email protected] Xavier CUCHERAT / Audinghen / [email protected] Benoît FONTAINE / Paris / [email protected] Olivier GARGOMINY / Paris / [email protected] Vincent PRIE / Montpellier / [email protected] Les manuscrits sont à envoyer à : Journal MalaCo Muséum national d’Histoire naturelle Equipe de Malacologie Case Postale 051 55, rue Buffon 75005 Paris Ou par Email à [email protected] MalaCo est téléchargeable gratuitement sur le site : http://www.journal-malaco.fr MalaCo (ISSN 1778-3941) est une publication de l’association Caracol Association Caracol Route de Lodève 34700 Saint-Etienne-de-Gourgas JO Association n° 0034 DE 2003 Déclaration en date du 17 juillet 2003 sous le n° 2569 Journal électronique de la malacologie continentale française MalaCo Septembre 2006 ▪ numéro 3 Au total, 119 espèces et sous-espèces de mollusques, dont quatre strictement endémiques, sont recensées dans les différents habitats du Parc naturel du Mercantour (photos Olivier Gargominy, se reporter aux figures 5, 10 et 17 de l’article d’O. Gargominy & Th. Ripken). Sommaire Page 100 Éditorial Page 101 Actualités Page 102 Librairie Page 103 Brèves & News ▪ Endémisme et extinctions : systématique des Endodontidae (Mollusca, Pulmonata) de Rurutu (Iles Australes, Polynésie française) Gabrielle ZIMMERMANN ▪ The first annual meeting of Task-Force-Limax, Bünder Naturmuseum, Chur, Switzerland, 8-10 September, 2006: presentation, outcomes and abstracts Isabel HYMAN ▪ Collecting and transporting living slugs (Pulmonata: Limacidae) Isabel HYMAN ▪ A List of type specimens of land and freshwater molluscs from France present in the national molluscs collection of the Hebrew University of Jerusalem Henk K. -

Gastropoda, Pleuroceridae), with Implications for Pleurocerid Conservation
Zoosyst. Evol. 93 (2) 2017, 437–449 | DOI 10.3897/zse.93.14856 museum für naturkunde Genetic structuring in the Pyramid Elimia, Elimia potosiensis (Gastropoda, Pleuroceridae), with implications for pleurocerid conservation Russell L. Minton1, Bethany L. McGregor2, David M. Hayes3, Christopher Paight4, Kentaro Inoue5 1 Department of Biological and Environmental Sciences, University of Houston Clear Lake, 2700 Bay Area Boulevard MC 39, Houston, Texas 77058 USA 2 Florida Medical Entomology Laboratory, Institute of Food and Agricultural Sciences, University of Florida, 200 9th Street SE, Vero Beach, Florida 32962 USA 3 Department of Biological Sciences, Eastern Kentucky University, 521 Lancaster Avenue, Richmond, Kentucky 40475 USA 4 Department of Biological Sciences, University of Rhode Island, 100 Flagg Road, Kingston, Rhode Island 02881 USA 5 Texas A&M Natural Resources Institute, 578 John Kimbrough Boulevard, 2260 TAMU, College Station, Texas 77843 USA http://zoobank.org/E6997CB6-F054-4563-8C57-6C0926855053 Corresponding author: Russell L. Minton ([email protected]) Abstract Received 7 July 2017 The Interior Highlands, in southern North America, possesses a distinct fauna with nu- Accepted 19 September 2017 merous endemic species. Many freshwater taxa from this area exhibit genetic structuring Published 15 November 2017 consistent with biogeography, but this notion has not been explored in freshwater snails. Using mitochondrial 16S DNA sequences and ISSRs, we aimed to examine genetic struc- Academic editor: turing in the Pyramid Elimia, Elimia potosiensis, at various geographic scales. On a broad Matthias Glaubrecht scale, maximum likelihood and network analyses of 16S data revealed a high diversity of mitotypes lacking biogeographic patterns across the range of E. -

The Abundant Occurrence of the Three-Toothed Bulin Snail Chondrula Tridens (O
Przegląd Przyrodniczy XXVII, 2 (2016): 87-94 Joanna Przybylska, Andrzej Jermaczek LICZNE WYSTĘPOWANIE WAŁKÓWKI TRÓJZĘBNEJ CHONDRULA TRIDENS (O. F. MÜLLER, 1774) WZDŁUŻ LINII KOLEJOWEJ OLEŚNICA – GNIEZNO W WIELKOPOLSCE The abundant occurrence of the Three-toothed Bulin Snail Chondrula tridens (O. F. Müller, 1774) along the Oleśnica – Gniezno railway in Wielkopolska ABSTRAKT: W artykule scharakteryzowano stanowiska kserotermicznego ślimaka – wałkówki trój- zębnej Chondrula tridens (O. F. Müller, 1774) – zlokalizowane w siedliskach antropogenicznych na nasypach i w wykopach linii kolejowej Oleśnica – Gniezno (Wielkopolska). W 2015 roku gatunek stwierdzono na 13 stanowiskach na odcinku ok. 100 km pomiędzy Zdunami a Gnieznem. Niewielki udział dogodnych siedlisk naturalnych w otoczeniu linii wskazuje, że występowanie gatunku na przy- torzach jest prawdopodobnie efektem zawleczenia wraz z materiałami budowlanymi. Otwarte, suche i nasłonecznione siedliska wzdłuż linii kolejowej stanowią dogodne siedliska zastępcze tego zagrożone- go gatunku mięczaka. SŁOWA KLUCZOWE: wałkówka trójzębna, linie kolejowe, siedliska antropogeniczne ABSTRACT: The paper presents the sites of the Three-toothed Bulin Snail Chondrula tridens (O. F. Müller, 1774) localised in anthropogenic habitats on embankments and in excavations along the Oleś- nica – Gniezno railway (Wielkopolska). In 2015 the species was found in 13 localities on a 100 km long section of the railway between Zduny and Gniezno. As natural habitats of the species in the sur- roundings are scarce, it is probable that the snails were introduced to the sites with building materials. Open, dry, insulated grasslands along tracks are suitable alternative habitats for this endangered snail species. KEY WORDS: Three-toothed Bulin Snail, railways, anthropogenic habitats Wstęp dopodobną rolę takich siedlisk i związanej z ich kształtowaniem działalności człowieka w Celem artykułu jest wskazanie na liczne rozprzestrzenianiu się gatunku. -

Diplom-Biologe KLAUS GROH Malakozoologe Und Naturschützer – 65 Jahre
53 Mitt. dtsch. malakozool. Ges. 94 53 – 70 Frankfurt a. M., November 2015 Diplom-Biologe KLAUS GROH Malakozoologe und Naturschützer – 65 Jahre CARSTEN RENKER & JÜRGEN H. JUNGBLUTH th Abstract: The 65 birthday of KLAUS GROH is a good occasion to give a retrospect of his life and hitherto existing achievement. Beside his vita we summarize his malacological work, give an overview about the projects for the protection of species, have a look on his tremendous impetus for the worldwide distribution of malacological knowledge by the establishment of the CHRISTA HEMMEN-Verlag, later ConchBooks, as publishing house, book trader and antiquarian. Last but not least we give a summary of his scientific achievements culminating in 206 publications and containing descriptions of up to now 42 specific taxa. Keywords: KLAUS GROH, biography, bibliography, malacology, freshwater mussels, Hesse, Rhineland- Palatinate, Luxembourg Zusammenfassung: Der 65. Geburtstag von KLAUS GROH wird zum Anlass genommen einen Rückblick auf sein bisheriges Leben und Wirken zu geben. Neben der Vita werden vor allem seine malakologische Arbeit und sein ehrenamtliches Engagement in zahlreichen malakologischen Verbänden und Naturschutzvereinen betrachtet. KLAUS GROH nahm außerdem einen enormen Einfluss auf die weltweite Verbreitung malako- logischen Wissens durch die Gründung des CHRISTA HEMMEN-Verlags, später ConchBooks, als Verlagshaus, Buchhandlung und Antiquariat. Schließlich gilt es seine wissenschaftlichen Verdienste zu würdigen, die in 206 Publikationen und Neubeschreibungen 42 spezifischer Taxa kulminieren. Vita Schulzeit Am 22. Mai 1949 wurde KLAUS GROH in Darmstadt als Sohn des Bauschlossers HELMUT GROH und seiner Ehefrau ANNELIESE, geb. FEDERLEIN geboren. Er besuchte die Volksschulen in Langen/Hessen und Kirchheim unter Teck/Baden-Württemberg (1955-1959), es folgte der Besuch der Realschule in Langen/Hessen (1959-1965), dort schloss er auch seine Schulzeit mit der „Mittleren Reife“ ab. -
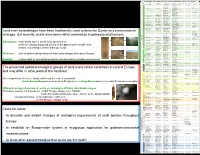
Ecological Groups of Snails – Use and Perspectives
The subdivision of all central European Holocene and Late Glacial land snail species to ecological groups ecological Glacial Early Holocene Middle Holocene Late Holocene (sensu Walker at al 2012) modern immigrants comment group Acanthinula aculeata Acanthinula aculeata Acanthinula aculeata Acanthinula aculeata Acicula parcelineata Acicula parcelineata Aegopinella epipedostoma one sites Aegopinella nitens Aegopinella nitens Aegopinella nitidula Aegopinella nitidula few sites Aegopinella pura Aegopinella pura Aegopinella pura Aegopinella pura Aegopis verticillus Ecological groups of snails Argna bielzi Argna bielzi Bulgarica cana Bulgarica cana Carpathica calophana Carpathica calophana one site; undated Causa holosericea Causa holosericea Clausilia bidentata no fossil data Clausilia cruciata Clausilia cruciata Clausilia cruciata – use and perspectives Cochlodina laminata Cochlodina laminata Cochlodina laminata Cochlodina laminata Cochlodina orthostoma Cochlodina orthostoma Cochlodina orthostoma Cochlodina orthostoma Daudebardia brevipes Daudebardia brevipes Daudebardia rufa Daudebardia rufa Daudebardia rufa Daudebardia rufa Discus perspectivus Discus perspectivus Discus perspectivus 1 2 1 1 ) Lucie Juřičková , Michal Horsák , Jitka Horáčková and Vojen Ložek Discus ruderatus Discus ruderatus Discus ruderatus Discus ruderatus Ena montana Ena montana Ena montana Ena montana forest Eucobresia nivalis Eucobresia nivalis Eucobresia nivalis Faustina faustina Faustina faustina Faustina faustina Faustina faustina Faustina rossmaessleri Faustina -

Canariella Planaria
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T156901A5014481 Canariella planaria Assessment by: Groh, K. & Neubert, E. View on www.iucnredlist.org Citation: Groh, K. & Neubert, E. 2013. Canariella planaria. The IUCN Red List of Threatened Species 2013: e.T156901A5014481. http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T156901A5014481.en Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: BirdLife International; Botanic Gardens Conservation International; Conservation International; Microsoft; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; Wildscreen; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Mollusca Gastropoda Stylommatophora Hygromiidae Taxon Name: Canariella planaria (Lamarck, 1822) Assessment Information Red List Category & Criteria: Least Concern ver 3.1 Year Published: 2013 Date Assessed: March 24, 2011 Justification: This species is endemic to northeastern coastal area of the island of Tenerife. -

Gastropoda, Pulmonata, Helicidae)
Ruthenica, 2012, vol. 22, No. 2: 93-100. © Ruthenica, 2012 Published October 20, 2012 http: www.ruthenica.com On the origin of Cochlopupa (= Cylindrus auct.) obtusa (Gastropoda, Pulmonata, Helicidae) Anatoly A. SCHILEYKO A.N. Severtzov Institute of Ecology and Evolution of Russian Academy of Sciences, Leninsky Prospect 33, 119071, Moscow, RUSSIA; e-mail: [email protected] ABSTRACT. Land snail Cochlopupa obtusa (Drapar- py) is widely-used for the species of Helicidae dur- naud, 1805) is an endemic of Eastern Alps. The mol- ing many tens of years. Unfortunately, this name is lusk has very unusual for helicids pupilloid shell, but invalid because it is junior homonym of Cylindrus its reproductive tract is quite typical for the subfamily Batsch, 1789 (Gastropoda, Conidae) (type species Ariantinae (Helicidae). Neither this species nor any Conus textile Linnaeus, 1758, by subsequent desig- similar forms are totally absent in fossil deposits (the nation Dubois and Bour, 2010). earliest records of C. obtusa conventionally dated by the “pre-Pleistocene”). According to suggested hy- At the same time the generic name Cylindrus pothesis, this species is very young and was formed Fitzinger is a junior objective synonym of Cochlo- within the existing area at the end of Würm glaciation pupa Jan, 1830 with type species Pupa obtusa due to mutation of some representative of Ariantinae. Draparnaud, 1805 (by monotypy). Thus, the correct binomen for the representative of Helicidae in ac- cordance with the rules of the ICZN must be Coch- Introduction lopupa obtusa (Draparnaud, 1805). Some years ago I have written that I directed a In Eastern Alps, on the territory of Austria, lives petition to the International Commission on Zoo- a very peculiar species of the Helicidae family – logical Nomenclature where I ask to conserve the Cochlopupa obtusa (Draparnaud, 1805) (Fig.