Impact Assessment of Intense Sport Climbing on Limestone Cliffs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Mollusca, Gastropoda) of the Bulgarian Part of the Alibotush Mts
Malacologica Bohemoslovaca (2008), 7: 17–20 ISSN 1336-6939 Terrestrial gastropods (Mollusca, Gastropoda) of the Bulgarian part of the Alibotush Mts. IVAILO KANEV DEDOV Central Laboratory of General Ecology, 2 Gagarin Str., BG-1113 Sofia, Bulgaria, e-mail: [email protected] DEDOV I.K., 2008: Terrestrial gastropods (Mollusca, Gastropoda) of the Bulgarian part of the Alibotush Mts. – Malacologica Bohemoslovaca, 7: 17–20. Online serial at <http://mollusca.sav.sk> 20-Feb-2008. This work presents results of two years collecting efforts within the project “The role of the alpine karst area in Bulgaria as reservoir of species diversity”. It summarizes distribution data of 44 terrestrial gastropods from the Bulgarian part of Alibotush Mts. Twenty-seven species are newly recorded from the Alibotush Mts., 13 were con- firmed, while 4 species, previously known from the literature, were not found. In the gastropod fauna of Alibotush Mts. predominate species from Mediterranean zoogeographic complex. A large part of them is endemic species, and this demonstrates the high conservation value of large limestone areas in respect of terrestrial gastropods. Key words: terrestrial gastropods, distribution, Alibotush Mts., Bulgaria Introduction Locality 6: vill. Katuntsi, Izvorite hut, near hut, open The Alibotush Mts. (other popular names: Kitka, Gotseva ruderal terrain, under bark, 731 m a.s.l., coll. I. Dedov. Planina, Slavjanka) is one of the most interesting large Locality 7: vill. Katuntsi, tufa-gorge near village, 700 m limestone area in Bulgaria (Fig. 1). It occupies the part a.s.l., coll. I. Dedov, N. Simov. of the border region between Bulgaria and Greece with Locality 8: below Livade area, road between Goleshevo maximum elevation 2212 m (Gotsev peak). -

Genus Cochlostoma, Subgenus Titanopoma (Gastropoda, Caenogastropoda, Cochlostomatidae)
BASTERIA, 68: 25-M A revision of the genus Cochlostoma, subgenus Titanopoma (Gastropoda, Caenogastropoda, Cochlostomatidae), in particular the forms occurring in Albania Zoltán Fehér H-1088 Baross Hungarian Natural History Museum, u. 13., Budapest, Hungary; [email protected] TitanopomaA. J. Wagner, 1897, a subgenus ofCochlostoma Jan, 1830, is revised on the basis of the in Natural Museum material the Hungarian History (Budapest), the Natural History Museum of the Humboldt University (Berlin) and the Zoological Institute and Museum of the Polish Academy of Sciences (Warsaw). Two species and five subspecies are described as new to sci- Shells and Because its relevance in ence. opercula are illustrated. of for species recognition this the of the is described in detail than usual. records and group, structure opercula more Locality some new zoogeographicaldata are given and mapped. Keywords: Cochlostoma, Titanopoma,Albania, Montenegro, Balkans, taxonomy, opercula, zoo- geography. INTRODUCTION is Cochlostoma Jan, 1830, a characteristic genus of rock-dwelling land snails of the Mediterranean region. Its subgenus Titanopoma A. J. Wagner, 1897, differs from the other the subgenera by structure of the operculum. The species are distributed in Montenegro and Albania (fig. 1). The Montenegrian part of the subgeneric range is relatively well explored (e.g. Wohlberedt, 1909; Sturany & Wagner, 1915; Wagner, 1897, 1906; Varga, 1998). However, there are hardly any data for Albania (e.g. Wohlberedt, 1909; Sturany & Wagner, 1915; Polinski, 1922, 1924; Welter-Schultes, 1996; Feher et al., 2001). Since the early 1990's, as soon as the political situation allowed it, a long-term mala- co-faunistical investigation is executed in Albania by staff members of the Hungarian Natural While the History Museum. -

Malaco Le Journal Électronique De La Malacologie Continentale Française
MalaCo Le journal électronique de la malacologie continentale française www.journal-malaco.fr MalaCo (ISSN 1778-3941) est un journal électronique gratuit, annuel ou bisannuel pour la promotion et la connaissance des mollusques continentaux de la faune de France. Equipe éditoriale Jean-Michel BICHAIN / Paris / [email protected] Xavier CUCHERAT / Audinghen / [email protected] Benoît FONTAINE / Paris / [email protected] Olivier GARGOMINY / Paris / [email protected] Vincent PRIE / Montpellier / [email protected] Les manuscrits sont à envoyer à : Journal MalaCo Muséum national d’Histoire naturelle Equipe de Malacologie Case Postale 051 55, rue Buffon 75005 Paris Ou par Email à [email protected] MalaCo est téléchargeable gratuitement sur le site : http://www.journal-malaco.fr MalaCo (ISSN 1778-3941) est une publication de l’association Caracol Association Caracol Route de Lodève 34700 Saint-Etienne-de-Gourgas JO Association n° 0034 DE 2003 Déclaration en date du 17 juillet 2003 sous le n° 2569 Journal électronique de la malacologie continentale française MalaCo Septembre 2006 ▪ numéro 3 Au total, 119 espèces et sous-espèces de mollusques, dont quatre strictement endémiques, sont recensées dans les différents habitats du Parc naturel du Mercantour (photos Olivier Gargominy, se reporter aux figures 5, 10 et 17 de l’article d’O. Gargominy & Th. Ripken). Sommaire Page 100 Éditorial Page 101 Actualités Page 102 Librairie Page 103 Brèves & News ▪ Endémisme et extinctions : systématique des Endodontidae (Mollusca, Pulmonata) de Rurutu (Iles Australes, Polynésie française) Gabrielle ZIMMERMANN ▪ The first annual meeting of Task-Force-Limax, Bünder Naturmuseum, Chur, Switzerland, 8-10 September, 2006: presentation, outcomes and abstracts Isabel HYMAN ▪ Collecting and transporting living slugs (Pulmonata: Limacidae) Isabel HYMAN ▪ A List of type specimens of land and freshwater molluscs from France present in the national molluscs collection of the Hebrew University of Jerusalem Henk K. -

Studien an Clausilia Dubia DRAPARNAUD 1805 (Stylommatophora: Clausiliidae)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Wissenschaftliche Mitteilungen Niederösterreichisches Landesmuseum Jahr/Year: 1997 Band/Volume: 10 Autor(en)/Author(s): Fellner (Frank) Christa Artikel/Article: Studien an Clausilia dubia DRAPARNAUD 1805 (Stylommatophora: Clausiliidae). (N.F. 417) 163-189 ©Amt der Niederösterreichischen Landesregierung,, download unter www.biologiezentrum.at Wiss. Mitt. Niederösterr. Landesmuseum 10 163 - 189 Wien 1997 Studien an Clausilia dubia DRAPARNAUD 1805 (Stylommatophora: Clausiliidae) CHRISTA FRANK Schlüsselwörter: Clausilia dubia, Pleistozän, subspezifische Gliederung Keywords: Clausilia dubia, Pleistocene, subspecific differentation Zusammenfassung Das pleistozäne Clausilia dwb/a-Material aus Höhlenfundstellen und Freiland- vorkommen Österreichs wird zum Anlaß genommen, die Frage nach der gegen- wärtigen reichen subspezifischen Gliederung dieser Art erneut aufzugreifen. Für die Aufgliederung der Linien dubia dubia und dubia speciosa muß der letzte Käl- tehöhepunkt der Würmvereisung auslösend gewesen sein. Die Vorläufer von Clausilia dubia dubia und Clausilia dubia speciosa zeigen morphologische Paral- lelen zu den Formen ostösterreichischer Lößfaunen, aus denen auch Clausilia dubia obsoleta hervorgegangen sein muß. Wenn die Beziehungen der beiden ersteren zueinander auch sehr eng sind, sollten doch alle drei Linien als eine Ein- heit angesehen werden. Summary The abundant pleistocene material of Clausilia dubia from different cave and loess localities in Austria made it possible to study its present subspecific dif- ferentiation. Presumably the last pleniglacial period of the alpine Wurmian glacia- tion induced the development of the two lines Clausilia dubia dubia and Clau- silia dubia speciosa. The ancestor forms of Clausilia dubia dubia and Clausilia dubia speciosa show morphological similarities to these dubia specimens which occur in a lot of loess localities in Eastern Austria. -

Draft Carpathian Red List of Forest Habitats
CARPATHIAN RED LIST OF FOREST HABITATS AND SPECIES CARPATHIAN LIST OF INVASIVE ALIEN SPECIES (DRAFT) PUBLISHED BY THE STATE NATURE CONSERVANCY OF THE SLOVAK REPUBLIC 2014 zzbornik_cervenebornik_cervene zzoznamy.inddoznamy.indd 1 227.8.20147.8.2014 222:36:052:36:05 © Štátna ochrana prírody Slovenskej republiky, 2014 Editor: Ján Kadlečík Available from: Štátna ochrana prírody SR Tajovského 28B 974 01 Banská Bystrica Slovakia ISBN 978-80-89310-81-4 Program švajčiarsko-slovenskej spolupráce Swiss-Slovak Cooperation Programme Slovenská republika This publication was elaborated within BioREGIO Carpathians project supported by South East Europe Programme and was fi nanced by a Swiss-Slovak project supported by the Swiss Contribution to the enlarged European Union and Carpathian Wetlands Initiative. zzbornik_cervenebornik_cervene zzoznamy.inddoznamy.indd 2 115.9.20145.9.2014 223:10:123:10:12 Table of contents Draft Red Lists of Threatened Carpathian Habitats and Species and Carpathian List of Invasive Alien Species . 5 Draft Carpathian Red List of Forest Habitats . 20 Red List of Vascular Plants of the Carpathians . 44 Draft Carpathian Red List of Molluscs (Mollusca) . 106 Red List of Spiders (Araneae) of the Carpathian Mts. 118 Draft Red List of Dragonfl ies (Odonata) of the Carpathians . 172 Red List of Grasshoppers, Bush-crickets and Crickets (Orthoptera) of the Carpathian Mountains . 186 Draft Red List of Butterfl ies (Lepidoptera: Papilionoidea) of the Carpathian Mts. 200 Draft Carpathian Red List of Fish and Lamprey Species . 203 Draft Carpathian Red List of Threatened Amphibians (Lissamphibia) . 209 Draft Carpathian Red List of Threatened Reptiles (Reptilia) . 214 Draft Carpathian Red List of Birds (Aves). 217 Draft Carpathian Red List of Threatened Mammals (Mammalia) . -

Format Mitteilungen
9 Mitt. dtsch. malakozool. Ges. 86 9 – 12 Frankfurt a. M., Dezember 2011 Under Threat: The Stability of Authorships of Taxonomic Names in Malacology RUUD A. BANK Abstract: Nomenclature must be constructed in accordance with agreed rules. The International Commission on Zoological Nomenclature was founded in Leiden in September 1895. It not only produced a Code of nomencla- ture, that was refined over the years, but also provided arbitration and advice service, all with the aim of ensur- ing that every animal has one unique and universally accepted name. Name changes reduce the efficiency of biological nomenclature as a reference system. The Code was established to precisely specify the circumstances under which a name must be changed, and in what way. Name changes are only permitted if it is necessitated by a correction of nomenclatural error, by a change in classification, or by a correction of a past misidentification. Also authorships are regulated by the Code, mainly by Article 50. In a recent paper by WELTER-SCHULTES this Article is interpreted in a way that is different from previous interpretations by the zoological (malacological) community, leading to major changes in authorships. It is here argued that his alternative interpretations (1) are not in line with the spirit of the Code, and (2) will not serve the stability of nomenclature. It is important that interpretation and application of the existing rules be objective, consistent, and clear. Keywords: authorships, malacology, nomenclature, Code, ICZN, Article 50, Pisidium Zusammenfassung: In der Nomenklatur müssen übereinstimmende Regeln gelten. Die Internationale Kommis- sion für Zoologische Nomenklatur (ICZN) wurde im September 1895 in Leiden gegründet. -

Open Carboniferous Limestone Pavement Grike Microclimates in Great Britain and Ireland: Understanding the Present to Inform the Future
Open Carboniferous Limestone pavement grike microclimates in Great Britain and Ireland: understanding the present to inform the future Item Type Thesis or dissertation Authors York, Peter, J. Citation York, P, J. (2020). Open Carboniferous Limestone pavement grike microclimates in Great Britain and Ireland: understanding the present to inform the future (Doctoral dissertation). University of Chester, UK. Publisher University of Chester Rights Attribution-NonCommercial-NoDerivatives 4.0 International Download date 10/10/2021 01:26:52 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/623502 Open Carboniferous Limestone pavement grike microclimates in Great Britain and Ireland: understanding the present to inform the future Thesis submitted in accordance with the requirements of the University of Chester for the degree of Doctor of Philosophy By Peter James York April 2020 I II Abstract Limestone pavements are a distinctive and irreplaceable geodiversity feature, in which are found crevices known as grikes. These grikes provide a distinct microclimate conferring a more stable temperature, higher relative humidity, lower light intensity and lower air speed than can be found in the regional climate. This stability of microclimate has resulted in an equally distinctive community of flora and fauna, adapted to a forest floor but found in an often otherwise barren landscape. This thesis documents the long-term study of the properties of the limestone pavement grike in order to identify the extent to which the microclimate may sustain its distinctive biodiversity, to provide recommendations for future research which may lead to more effective management. Over a five-year study, recordings of temperature, relative humidity, light intensity and samples of invertebrate biodiversity were collected from five limestone pavements situated in the Yorkshire Dales and Cumbria in Great Britain, and The Burren in the Republic of Ireland. -
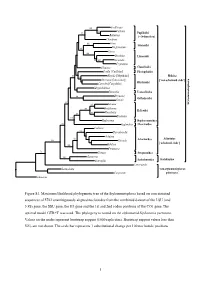
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Addendum to the Type Catalogue of the Malacological Collection in the Museu De Ciències Naturals De Barcelona
Arxius de Miscel·lània Zoològica, 16 (2018): 40–95 Martínez–OrtíISSN: 1698– et0476 al. Addendum to the type catalogue of the malacological collection in the Museu de Ciències Naturals de Barcelona A. Martínez–Ortí, M. Prieto, F. Uribe Martínez–Ortí, A., Prieto, M., Uribe, F., 2018. Addendum to the type catalogue of the mala- cological collection in the Museu de Ciències Naturals de Barcelona. Arxius de Miscel·lània Zoològica, 16: 40–95, Doi: https://doi.org/10.32800/amz.2018.16.0040 Abstract Addendum to the type catalogue of the malacological collection in the Museu de Ciències Naturals de Barcelona. The catalogue of type specimens of molluscs of the Museu de Ciències Naturals de Barcelona, published in 2008, is updated with new material corresponding to 42 species and subspecies of continental molluscs from the Iberian peninsula, the Balearic Islands, and the Canary Islands. Most of these type specimens belong to historical collec- tions of the museum or are part of the malacological collection of Miquel Bech, donated in 2009. Information provided includes the type category, number of specimens, geographic provenance, type specimens housed at other institutions, and current taxonomical status, together with supporting texts of nomenclatures if pertinent. The catalogue also includes a brief gallery of photographs of types per taxon. Key words: Catalogue, Type specimens, Continental molluscs, Museu de Ciències Naturals de Barcelona Resumen Nuevas aportaciones al catálogo de ejemplares tipo de la colección malacológica del Museu de Ciènciae Naturals de Barcelona. Se amplía el catálogo de ejemplares tipo de moluscos del Museu de Ciències Naturals de Barcelona, publicado en 2008, con nuevo material co- rrespondiente a 42 taxones de moluscos continentales procedentes de la península ibérica, islas Baleares y Canarias. -

An Annotated List of the Non-Marine Mollusca of Britain and Ireland
JOURNAL OF CONCHOLOGY (2005), VOL.38, NO .6 607 AN ANNOTATED LIST OF THE NON-MARINE MOLLUSCA OF BRITAIN AND IRELAND ROY ANDERSON1 Abstract An updated nomenclatural list of the non-marine Mollusca of the Britain and Ireland is provided. This updates all previous lists and revises nomenclature and classification in the context of recent changes and of new European lists, including the Clecom List. Cases are made for the usage of names in the List by means of annotations. The List will provide a basis for the future census and cataloguing of the fauna of Britain and Ireland. Key words Taxonomic, list, nomenclature, non-marine, Mollusca, Britain, Ireland, annotated. INTRODUCTION There has been a need for some time to modernise the list of non-marine Mollusca for Britain and Ireland, a subject last visited in this journal in 1976 (Waldén 1976; Kerney 1976). Many of the changes that have appeared in the literature since then are contentious and Kerney (1999) chose not to incorporate many of these into the latest atlas of non-marine Mollusca of Britain and Ireland. A new European List, the Clecom List (Falkner et al. 2001) has now appeared and it seems appropriate to examine in more detail constituent changes which might affect the British and Irish faunas. This is given additional urgency by the inception of a new census of the molluscs of Britain and Ireland by the Conchological Society. Recorders in the Society are aware of many of the proposed changes but unable to implement them without general agreement. In addition, many field malacologists make use of the recording package RECORDER, a recent form of which has been developed jointly by JNCC and the National Biodiversity Network in the United Kingdom. -

Molluscan Fauna of the Javoříčský Karst (Czech Republic, Central Moravia)
MALAKOLÓGIAI TÁJÉKOZTATÓ MALACOLOGICAL NEWSLETTER 2002 20: 93–105 Molluscan fauna of the Javoříčský Karst (Czech Republic, central Moravia) J. Č. Hlaváč Abstract: Data about Recent molluscan fauna of the Javoříčský Karst are given. Altogether 77 species (76 species of Gastropoda, 1 species of Bivalvia) were recorded. Molluscan fauna is dominated by wood- land communities occurring in various types of woodland habitats while elements of open grounds and aquatic habitats are poorly represented. Of prime importance is the occurrence of the Alpine and Carpathian elements at the same place as well as the records of sensitive woodland species Bulgarica cana and overlooked slug Deroceras turcicum, both new ones for the area of the Javoříčský Karst. The list of recorded molluscs is enclosed in this paper as well as the quadrangle mapping of important speci- es Orcula dolium and Itala ornata. Due to the confusion of epilithic species Chondrina avenacea, report- ed from the area studied in the end of 19th century, the distributions of Chondrina clienta and Chondrina avenacea in the Czech Republic are shortly commented and compared in the form of quadrangle maps. Key words: Czech Republic, Javoříčský Karst, list of species, Orcula dolium, Itala ornata, quadrangle mapping Introduction Several karst areas are present in the Czech Republic. Among them, the Bohemian Karst and Moravian Karst represent a typically developed karst phenomenon. Other, generally small karstic areas show poorly or partly developed karst phenomenon. The fragmentarily developed karst phenomenon is mostly characterised by subterranean features while surface ones are often absent. Although the Javoříčský Karst shows fragmentarily developed karst phenomenon, its molluscan fauna is rather varied, much like the malacofauna of the Bohemian or Moravian Karst. -

A List of the Land Snails (Mollusca: Gastropoda) of Croatia, with Recommendations for Their Croatian Names
NAT. CROAT. VOL. 19 No 1 1–76 ZAGREB June 30, 2010 original scientific paper/izvorni znanstveni rad A LIST OF THE LAND SNAILS (MOLLUSCA: GASTROPODA) OF CROATIA, WITH RECOMMENDATIONS FOR THEIR CROATIAN NAMES VESNA [TAMOL Department of Zoology, Croatian Natural History Museum, Demetrova 1, 10000 Zagreb, Croatia [tamol, V.: A list of the land snails (Mollusca: Gastropoda) of Croatia, with recommendations for their Croatian names. Nat. Croat., Vol. 19, No. 1, 1–76, 2010, Zagreb. By examination of extensive literature data, a list of the terrestrial snails of Croatia has been compiled. A list of Croatian names for each taxon is also provided for the first time. Croatian en- demic species and subspecies are indicated. Key words: land snails, Croatia, Croatian names, common names, endemics [tamol, V.: Popis kopnenih pu`eva (Mollusca: Gastropoda) Hrvatske s prijedlogom njihovih hrvatskih imena. Nat. Croat., Vol. 19, No. 1, 1–76, 2010, Zagreb. Obradom literaturnih podataka sastavljen je popis kopnenih pu`eva Hrvatske po prvi puta popra}en hrvatskim imenima svih svojti. Odre|ena je endemi~nost vrsta i podvrsta za Hrvatsku. Klju~ne rije~i: kopneni pu`evi, Hrvatska, hrvatska imena pu`eva, endemi INTRODUCTION This paper attempts to provide an overview of the species and subspecies of ter- restrial molluscs of Croatia. To date, lists have been compiled of the malacofauna of some regions (BRUSINA, 1866, 1870, 1907) which are in whole or in part within the political borders of today’s Croatia. The Croatian fauna was also included in the list of terrestrial and freshwater molluscs of the northern Balkans (JAECKEL et al., 1958), though this was spatially undefined as that publication divided present day Croatia into three regions, and unfortunately, only one of which (»Croatia«) is undoubtedly within the national borders today.