Microbat Paraphyly and the Convergent Evolution of a Key Innovation in Old World Rhinolophoid Microbats
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Meseret Chane Alemu Advisor
SCHOOL OF GRADUATE STUDIES DEPARTMENT OF ZOOLOGICAL SCIENCES Effect of Anthropogenic Habitat Modification on Behavioural Ecology of Boutourlini's Blue Monkey (Cercopithecus mitis boutourlinii Giglioli, 1887) in Hamuma Coffee Forest, Southwestern Ethiopia By: Meseret Chane Alemu Advisor: Professor Afework Bekele Addis Ababa, Ethiopia June 2020 ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES DEPARTMENT OF ZOOLOGICAL SCIENCES Effect of Anthropogenic Habitat Modification on Behavioural Ecology of Boutourlini's Blue Monkey (Cercopithecus mitis boutourlinii Giglioli, 1887) in Hamuma Coffee Forest, Southwestern Ethiopia By: Meseret Chane Alemu A Thesis Submitted to the School of Graduate Studies of the Addis Ababa University, Department of Zoological Sciences, in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology (Ecological and Systematic Zoology) Approved by Examining Committee: Name Signature 1. Professor Afework Bekele (Advisor)_____________________ 2. Prof./Dr. ____________________ (Examiner) ____________________ 3. Prof./Dr. ____________________ (Examiner) _____________________ 4. Dr. Bezawork Afework (Chairperson) ___________________ I hereby declare that this thesis, entitled: “Effect of Anthropogenic Habitat Modification on Behavioural Ecology of Boutourlini's Blue Monkey (Cercopithecus mitis boutourlinii Giglioli, 1887) in Hamuma Coffee Forest, Southwestern Ethiopia” has been composed entirely by myself and has not been submitted for any other degree or qualification. The work complies with the regulations of the University and meets the accepted standards with respect to originality and quality and all sources of information have been specifically acknowledged. Meseret Chane: Signature __________________ Date ____________________ ABSTRACT Effect of Anthropogenic Habitat Modification on Behavioural Ecology of Boutourlini's Blue Monkey (Cercopithecus mitis boutourlinii Giglioli, 1887) in Hamuma Coffee Forest, Southwestern Ethiopia Meseret Chane Alemu. -

Base-Compositional Biases and the Bat Problem. III. the Question of Microchiropteran Monophyly
Base-compositional biases and the bat problem. III. The question of microchiropteran monophyly James M. Hutcheon1,JohnA.W.Kirsch1 and John D. Pettigrew2 1The University of Wisconsin Zoological Museum, 250 North Mills Street, Madison,WI 53706, USA 2Vision,Touch, and Hearing Research Centre, University of Queensland, St Lucia, Queensland 4072, Australia Using single-copy DNA hybridization, we carried out a whole genome study of 16 bats (from ten families) and ¢ve outgroups (two primates and one each dermopteran, scandentian, and marsupial). Three of the bat species represented as many families of Rhinolophoidea, and these always associated with the two representatives of Pteropodidae. All other microchiropterans, however, formed a monophyletic unit displaying interrelationships largely in accord with current opinion. Thus noctilionoids comprised one clade, while vespertilionids, emballonurids, and molossids comprised three others, successively more closely related in that sequence. The unexpected position of rhinolophoids may be due either to the high AT bias they share with pteropodids, or it may be phylogenetically authentic. Reanalysis of the data with varying combinations of the ¢ve outgroups does not indicate a rooting problem, and the inclusion of many bat lineages divided at varying levels similarly discounts long branch attraction as an explanation for the pteropodid^rhinolophoid association. If rhinolophoids are indeed specially related to pteropodids, many synapomorphies of Microchiroptera are called into question, not least the -

Colugos: Obscure Mammals Glide Into the Evolutionary Limelight Robert D Martin
BioMed Central Minireview Colugos: obscure mammals glide into the evolutionary limelight Robert D Martin Address: Department of Anthropology, The Field Museum, Chicago, IL 60605-2496, USA. Email: [email protected] Published: 1 May 2008 Journal of Biology 2008, 7:13 (doi:10.1186/jbiol74) The electronic version of this article is the complete one and can be found online at http://jbiol.com/content/7/4/13 © 2008 BioMed Central Ltd Abstract Substantial molecular evidence indicates that tree-shrews, colugos and primates cluster together on the mammalian phylogenetic tree. Previously, a sister-group relationship between colugos and primates seemed likely. A new study of colugo chromosomes indicates instead an affinity between colugos and tree-shrews. Colugos, constituting the obscure and tiny order Dermop- shrews, tree-shrews, colugos, bats and primates. However, tera, are gliding mammals confined to evergreen tropical Simpson’s ensuing influential classification of mammals [1] rainforests of South-East Asia. There are two extant species, rejected this assemblage. Subsequently, prompted by Butler now placed in separate genera: Galeopterus variegatus [4], the superorder Archonta was progressively resuscitated, (Malayan colugo, formerly known as Cynocephalus variegatus) although most authors emphatically excluded elephant- and Cynocephalus volans (Philippine colugo). Their most shrews (for example [5,6]). A quite recent major classifi- obvious hallmark is a gliding membrane (patagium) cation of mammals [7] united tree-shrews, colugos, bats surrounding almost the entire body margin. Colugos are and primates in the grand order Archonta. also called ‘flying lemurs’, but - as Simpson aptly noted [1] - they “are not lemurs and cannot fly”. They differ from other This whole topic has been reinvigorated by molecular gliding mammals (certain rodents and marsupials) in that evidence indicating that tree-shrews, colugos and primates, the patagium also extends between the hind limbs and the at least, may be quite closely related. -

NEOTROPICAL Primates VOLUME 9 NUMBER 3 DECEMBER 2001
ISSN 1413-4703 NEOTROPICAL primates VOLUME 9 NUMBER 3 DECEMBER 2001 A Journal and Newsletter of the Neotropical Section of the IUCN/SSC Primate Specialist Group Editors: Anthony B. Rylands and Ernesto Rodríguez-Luna PSG Chairman: Russell A. Mittermeier PSG Deputy Chairmen: Anthony B. Rylands and William R. Konstant Neotropical Primates A Journal and Newsletter of the Neotropical Section of the IUCN/SSC Primate Specialist Group Center for Applied Biodiversity Science S Conservation International T 1919 M. St. NW, Suite 600, Washington, DC 20036, USA t ISSN 1413-4703 w Abbreviation: Neotrop. Primates a Editors t Anthony B. Rylands, Center for Applied Biodiversity Science, Conservation International, Washington, DC Ernesto RodrÌguez-Luna, Universidad Veracruzana, Xalapa, Mexico S Assistant Editor Jennifer Pervola, Center for Applied Biodiversity Science, Conservation International, Washington, DC P P Editorial Board Hannah M. Buchanan-Smith, University of Stirling, Stirling, Scotland, UK B Adelmar F. Coimbra-Filho, Academia Brasileira de CiÍncias, Rio de Janeiro, Brazil D Liliana CortÈs-Ortiz, Universidad Veracruzana, Xalapa, Mexico < Carolyn M. Crockett, Regional Primate Research Center, University of Washington, Seattle, WA, USA t Stephen F. Ferrari, Universidade Federal do Par·, BelÈm, Brazil Eckhard W. Heymann, Deutsches Primatenzentrum, Gˆttingen, Germany U William R. Konstant, Conservation International, Washington, DC V Russell A. Mittermeier, Conservation International, Washington, DC e Marta D. Mudry, Universidad de Buenos Aires, Argentina Hor·cio Schneider, Universidade Federal do Par·, BelÈm, Brazil Karen B. Strier, University of Wisconsin, Madison, Wisconsin, USA C Maria EmÌlia Yamamoto, Universidade Federal do Rio Grande do Norte, Natal, Brazil M Primate Specialist Group a Chairman Russell A. Mittermeier Deputy Chairs Anthony B. -

Eutheria (Placental Mammals) Thought of As More Primitive
Eutheria (Placental Introductory article Mammals) Article Contents . Introduction J David Archibald, San Diego State University, San Diego, California, USA . Basic Design . Taxonomic and Ecological Diversity Eutheria includes one of three major clades of mammals, the extant members of which are . Fossil History and Distribution referred to as placentals. Phylogeny Introduction doi: 10.1038/npg.els.0004123 Eutheria (or Placentalia) is the most taxonomically diverse each. Except for placentals that have supernumerary teeth of three branches or clades of mammals, the other two (e.g. some whales, armadillos, etc.), in extant placentals, the being Metatheria (or Marsupialia) and Prototheria (or number of teeth is at most three upper and lower incisors, Monotremata). When named by Gill in 1872, Eutheria in- one upper and lower canine, four upper and lower premo- cluded both marsupials and placentals. It was Huxley in lars and three upper and lower molars. Pigs retain this pat- 1880 who recognized Eutheria basically as used today to tern, and except for one fewer upper molar, a domestic dog include only placentals. McKenna and Bell in their Clas- does as well. Compared to reptiles, mammals have fewer sification of Mammals published in 1997, chose to use Pla- skull bones through fusion and loss, although bones are centalia rather than Eutheria to avoid the confusion of variously emphasized in each of the three major mammalian what taxa should be included in Eutheria. Others such as taxa. See also: Digestive system of mammals; Ingestion in Rougier have used Eutheria and Placentalia in the sense mammals; Mesozoic mammals; Reptilia (reptiles) used here. Placentalia includes all extant placentals and Physiologically, mammals are all endotherms with var- their most recent common ancestor. -

The Soft Explosive Model of Placental Mammal Evolution
bioRxiv preprint doi: https://doi.org/10.1101/251520; this version posted January 22, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The soft explosive model of placental mammal evolution Matthew J. Phillips*,1 and Carmelo Fruciano1 1School of Earth, Environmental and Biological Sciences, Queensland University of Technology, Brisbane, Australia *Corresponding author: E-mail: [email protected] Abstract Recent molecular dating estimates for placental mammals echo fossil inferences for an explosive interordinal diversification, but typically place this event some 10-20 million years earlier than the Paleocene fossils, among apparently more “primitive” mammal faunas. However, current models of molecular evolution do not adequately account for parallel rate changes, and result in dramatic divergence underestimates for large, long-lived mammals such as whales and hominids. Calibrating among these taxa shifts the rate model errors deeper in the tree, inflating interordinal divergence estimates. We employ simulations based on empirical rate variation, which show that this “error-shift inflation” can explain previous molecular dating overestimates relative to fossil inferences. Molecular dating accuracy is substantially improved in the simulations by focusing on calibrations for taxa that retain plesiomorphic life-history characteristics. Applying this strategy to the empirical data favours the soft explosive model of placental evolution, in line with traditional palaeontological interpretations – a few Cretaceous placental lineages give rise to a rapid interordinal diversification following the 66 Ma Cretaceous-Paleogene boundary mass extinction. -

New Postcranial Bones of the Extinct Mammalian Family Nyctitheriidae (Paleogene, UK): Primitive Euarchontans with Scansorial Locomotion
Palaeontologia Electronica palaeo-electronica.org New postcranial bones of the extinct mammalian family Nyctitheriidae (Paleogene, UK): Primitive euarchontans with scansorial locomotion Jerry J. Hooker ABSTRACT New postcranial bones from the Late Eocene and earliest Oligocene of the Hamp- shire Basin, UK, are identified as belonging to the extinct family Nyctitheriidae. Previ- ously, astragali and calcanea were the only elements apart from teeth and jaws to be unequivocally recognized. Now, humeri, radii, a metacarpal, femora, distal tibiae, naviculars, cuboids, an ectocuneiform, metatarsals and phalanges, together with addi- tional astragali and calcanea, have been collected. These are shown to belong to a diversity of nyctithere taxa previously named from dental remains. Functional analysis shows that nyctitheres had mobile shoulder and hip joints, could pronate and supinate the radius, partially invert the foot at the astragalocalcaneal and upper ankle joints using powerful flexor muscles, all indicative of a scansorial lifestyle and allowing head- first descent on vertical surfaces. Climbing appears to have been dominated by flexion of the forearms and feet. Cladistic analysis employing a range of primitive eutherian mammals shows that nyctitheres are stem euarchontans, rather than lipotyphlans, with which they had previously been classified based on dental characters. Earlier ideas of relationships with the extinct Adapisoriculidae, recently considered stem eutherians, are not upheld. Jerry J. Hooker. Department of Earth Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK. [email protected] Keywords: Eutheria; Lipotyphla; placental; Scandentia; shrews; Soricidae INTRODUCTION basis of some straight slender limb bone shafts, apparently associated with teeth (on which this Nyctitherium Marsh, 1872, the type genus of genus was based), returned to Marsh’s original the eutherian family Nyctitheriidae Simpson, 1928a idea that Nyctitherium was a chiropteran. -

Diplomarbeit
DIPLOMARBEIT Titel der Diplomarbeit Annotated catalogue of primate type specimens in the mammal collection of the Museum of Natural History Vienna angestrebter akademischer Grad Magister der Naturwissenschaften (Mag. rer.nat.) Verfasserin / Verfasser: Simon Engelberger Studienrichtung /Studienzweig Biologie/Zoologie (lt. Studienblatt): Betreuerin / Betreuer: Univ.-Prof. Dr. Ulrike Aspöck Wien, im Mai 2010 Table of contents 1. Abstract...................................................................................................................1 2. Introduction.............................................................................................................1 2.1. Primate evolution and systematics.......................................................................1 2.2. Current primate taxonomy...................................................................................4 2.3. History of Primate Taxonomy..............................................................................4 2.4. Importance of type specimens..............................................................................5 2.5. History and Documentation of the collection......................................................7 3. Methods.................................................................................................................10 4. Catalogue of type specimens.................................................................................12 4.1. Megaladapidae FORSYTH MAJOR, 1893.............................................................12 -

Oldest Known Euarchontan Tarsals and Affinities of Paleocene Purgatorius to Primates
Oldest known euarchontan tarsals and affinities of Paleocene Purgatorius to Primates Stephen G. B. Chestera,b,c,1, Jonathan I. Blochd, Doug M. Boyere, and William A. Clemensf aDepartment of Anthropology and Archaeology, Brooklyn College, City University of New York, Brooklyn, NY 11210; bNew York Consortium in Evolutionary Primatology, New York, NY 10024; cDepartment of Anthropology, Yale University, New Haven, CT 06520; dFlorida Museum of Natural History, University of Florida, Gainesville, FL 32611; eDepartment of Evolutionary Anthropology, Duke University, Durham, NC 27708; and fUniversity of California Museum of Paleontology, Berkeley, CA 94720 Edited by Neil H. Shubin, The University of Chicago, Chicago, IL, and approved December 24, 2014 (received for review November 12, 2014) Earliest Paleocene Purgatorius often is regarded as the geologi- directly outside Placentalia with the contemporary condylarths cally oldest primate, but it has been known only from fossilized (archaic ungulates) Protungulatum and Oxyprimus (10–12). How- dentitions since it was first described half a century ago. The den- ever, the addition of new tarsal data for Purgatorius and increased tition of Purgatorius is more primitive than those of all known taxon sampling, including a colugo and four plesiadapiforms, using living and fossil primates, leading some researchers to suggest this same matrix, results in a strict consensus tree that supports that it lies near the ancestry of all other primates; however, others a monophyletic Euarchonta with Sundatheria (treeshrews and have questioned its affinities to primates or even to placental colugos) as the sister group to a fairly unresolved Primates clade mammals. Here we report the first (to our knowledge) nondental that includes Purgatorius (Fig. -
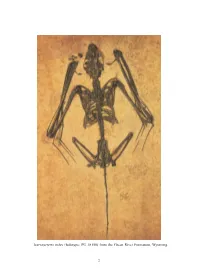
2 Icaronycteris Index
Icaronycteris index (holotype; PU 18150) from the Green River Formation, Wyoming. 2 CONTENTS Abstract ....................................................................... 4 Introduction .................................................................... 5 Relationships and Classi®cation of Eocene Bats: A Historical Overview ........... 12 Relationships Among Extant Lineages of Bats .................................. 31 Goals of the Current Study ................................................... 40 Materials and Methods ......................................................... 40 Taxonomic Sampling and Monophyly .......................................... 40 Outgroups .................................................................. 42 The Data Set ................................................................ 46 Characters Examined in Fossil Bats ............................................ 48 Skull and Dentition ........................................................ 48 Anterior Axial Skeleton .................................................... 64 Pectoral Girdle ............................................................ 70 Forelimb ................................................................. 77 Posterior Axial Skeleton and Pelvis .......................................... 81 Hindlimb ................................................................. 84 Completeness ............................................................... 85 Methods of Phylogenetic Analysis ............................................. 86 Analysis -

Brief Contents
Brief Contents hn hk io il sy SY ek eh hn hk io il sy SY ek eh hn hk io il sy SY ek eh hn hk io il sy SY ek eh Preface xii Chapter 17 Carnivora 352 hn hk io il sy SY ek eh Chapter 18 Rodentia and Lagomorpha 374 hn hk io il sy SY ek eh P arT 1 Chapter 19 Proboscidea, Hyracoidea, hn hk io il sy SY ek eh Introduction 1 and Sirenia 402 hn hk io il sy SY ek eh Chapter 20 Perissodactyla and Artiodactyla 418 hn hk io il sy SY ek eh Chapter 1 The Study of Mammals 2 Chapter 21 Cetacea 444 hn hk io il sy SY ek eh Chapter 2 History of Mammalogy 8 hn hk io il sy SY ek eh Chapter 3 Methods for Studying Mammals 22 Chapter 4 Phylogeny and Diversification of Mammals 50 P arT 4 Chapter 5 Evolution and Dental Behavior and Ecol ogy 467 Characteristics 62 Chapter 22 Communication, Aggression, Chapter 6 Biogeography 82 and Spatial Relations 468 Chapter 23 Sexual Selection, Parental Care, and Mating Systems 484 P arT 2 Chapter 24 Social Behavior 500 Structure and Function 109 Chapter 25 Dispersal, Habitat Selection, and Migration 514 Chapter 7 Integument, Support, Chapter 26 Populations and Life History 530 and Movement 110 Chapter 27 Community Ecol ogy 548 Chapter 8 Modes of Feeding 132 Chapter 9 Control Systems and Biological Rhythms 162 Chapter 10 Environmental Adaptations 176 P arT 5 Chapter 11 Reproduction 214 Special Topics 569 Chapter 28 Parasites and Diseases 570 Chapter 29 Domestication and P arT 3 Domesticated Mammals 590 Adaptive Radiation and Diversity 237 Chapter 30 Conservation 604 Chapter 12 Monotremes and Marsupials 242 Glossary 624 Chapter -

Fossil Evidence and the Origin of Bats
Journal of Mammalian Evolution, Vol. 12, Nos. 1/2, June 2005 (C 2005) DOI: 10.1007/s10914-005-6945-2 Fossil Evidence and the Origin of Bats Gregg F. Gunnell1,3 and Nancy B. Simmons2 The phylogenetic and geographic origins of bats (Chiroptera) remain unknown. The earliest con- firmed records of bats date from the early Eocene (approximately 51 Ma) in North America with other early Eocene bat taxa also being represented from Europe, Africa, and Australia. Where known, skeletons of these early taxa indicate that many of the anatomical specializations char- acteristic of bats had already been achieved by the early Eocene, including forelimb and manus elongation in conjunction with structural changes in the pectoral skeleton, hind limb reorientation, and the presence of rudimentary echolocating abilities. By the middle Eocene, the diversification of bats was well underway with many modern families being represented among fossil forms. A new phylogenetic analysis indicates that several early fossil bats are consecutive sister taxa to the extant crown group (including megabats), and suggests a single origin for the order, at least by the late Paleocene. Although morphological studies have long placed bats in the Grandorder Archonta, (along with primates dermopterans, and tree shrews), recent molecular studies have refuted this hypothesis, instead strongly supporting placement of bats in Laurasiatheria. Primitively, proto-bats were likely insectivorous, under-branch hangers and elementary gliders that exploited terminal branch habitats. Recent work has indicated that a number of other mammalian groups began to exploit similar arboreal, terminal branch habitats in the Paleocene, including multituberculates, eulipotyphlans, dermopterans, and plesiadapiforms.