Fossil Evidence and the Origin of Bats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dating Placentalia: Morphological Clocks Fail to Close the Molecular-Fossil Gap
Puttick, M. N., Thomas, G. H., & Benton, M. J. (2016). Dating placentalia: Morphological clocks fail to close the molecular-fossil gap. Evolution, 70(4), 873-886. https://doi.org/10.1111/evo.12907 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1111/evo.12907 Link to publication record in Explore Bristol Research PDF-document © 2016 The Author(s). Evolution published by Wiley Periodicals, Inc. on behalf of The Society for the Study of Evolution. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ ORIGINAL ARTICLE doi:10.1111/evo.12907 Dating placentalia: Morphological clocks fail to close the molecular fossil gap Mark N. Puttick,1,2 Gavin H. Thomas,3 and Michael J. Benton1 1School of Earth Sciences, Life Sciences Building, Tyndall Avenue, University of Bristol, Bristol, BS8 1TQ, United Kingdom 2E-mail: [email protected] 3Department of Animal and Plant Sciences, Alfred Denny Building, University of Sheffield, Western Bank, Sheffield, S10 2TN, United Kingdom Received January 31, 2015 Accepted March 7, 2016 Dating the origin of Placentalia has been a contentious issue for biologists and paleontologists. -

Microchiroptera: Mystacinidae) from Australia, with a Revised Diagnosis of the Genus
New Miocene Icarops material (Microchiroptera: Mystacinidae) from Australia, with a revised diagnosis of the genus SUZANNE HAND, MICHAEL ARCHER & HENK GODTHELP HAND, S.l., ARCHER, M. & GODTHELP, H., 2001:12:20. New Miocene lcarops material (Microchiroptera: Mystacinidae) from Australia, with a revised diagnosis of the genus. Memoirs of the Association of Australasian Palaeontologists 25,139-146. ISSN 0810-8889 New fossil material referable to Icarops paradox Hand et al., 1998 is described from the early Miocene Judith's Horizontalis Site in the Riversleigh World Heritage Property of northwestern Queensland. Fused dentaries contain the partial lower dentition of I. paradox. The diagnosis of the genus Icarops is revised. The new material confirms the identity of Icarops species as mystacinids and enablesre-examination of interrelationships between extinct and extant members of this Gondwanan bat family. S.J: Hand, M. Archer* & H. Godthelp, School of Biological Science, University of New South Wales,New South Wales, 2052; * also Australian Museum, 6-8 College St, Sydney, New South Wales,2000. Received ]4 December 2000 Keywords: Mystacinidae, Icarops, Mystacina, bat, lower dentition, Miocene, Riversleigh THE FIRST pre-Pleistocene record for the QMF refers to specimens held in the fossil Mystacinidae and first record of this bat family collections of the QueenslandMuseum, Brisbane. from outside New Zealand were reported by Hand et al. ( 1998) from Miocene sedimentsin Australia. SYSTEMAllC PALAEONTOLOGY Three species of the new mystacinid genus Icarops were described: Icarops breviceps from OrderCIllROPTERAB1wnenbach, 1779 the middle Miocene Bullock Creek deposit of the SuborderMICROCIllROPTERA Dobson, 1875 Northern Territory; I. aenae from the early SuperfamilyNocmIoNoIDEA Van Va1en, Miocene Wayne's Wok deposit, D Site Plateau, 1979 Riversleigh, northwestern Queensland; and I. -

Diversity and Abundance of Roadkilled Bats in the Brazilian Atlantic Forest
diversity Article Diversity and Abundance of Roadkilled Bats in the Brazilian Atlantic Forest Lucas Damásio 1,2 , Laís Amorim Ferreira 3, Vinícius Teixeira Pimenta 3, Greiciane Gaburro Paneto 4, Alexandre Rosa dos Santos 5, Albert David Ditchfield 3,6, Helena Godoy Bergallo 7 and Aureo Banhos 1,3,* 1 Centro de Ciências Exatas, Naturais e da Saúde, Departamento de Biologia, Universidade Federal do Espírito Santo, Alto Universitário, s/nº, Guararema, Alegre 29500-000, ES, Brazil; [email protected] 2 Programa de Pós-Graduação em Ecologia, Instituto de Ciências Biológicas, Campus Darcy Ribeiro, Universidade de Brasília, Brasília 70910-900, DF, Brazil 3 Programa de Pós-Graduação em Ciências Biológicas (Biologia Animal), Universidade Federal do Espírito Santo, Av. Fernando Ferrari, 514, Prédio Bárbara Weinberg, Vitória 29075-910, ES, Brazil; [email protected] (L.A.F.); [email protected] (V.T.P.); [email protected] (A.D.D.) 4 Centro de Ciências Exatas, Naturais e da Saúde, Departamento de Farmácia e Nutrição, Universidade Federal do Espírito Santo, Alto Universitário, s/nº, Guararema, Alegre 29500-000, ES, Brazil; [email protected] 5 Centro de Ciências Agrárias e Engenharias, Departamento de Engenharia Rural, Universidade Federal do Espírito Santo, Alto Universitário, s/nº, Guararema, Alegre 29500-000, ES, Brazil; [email protected] 6 Centro de Ciências Humanas e Naturais, Departamento de Ciências Biológicas, Universidade Federal do Espírito Santo, Av. Fernando Ferrari, 514, Vitória 29075-910, ES, Brazil 7 Departamento de Ecologia, Instituto de Biologia Roberto Alcântara Gomes, Universidade do Estado do Rio de Janeiro, Rua São Francisco Xavier 524, Maracanã, Rio de Janeiro 20550-900, RJ, Brazil; [email protected] Citation: Damásio, L.; Ferreira, L.A.; * Correspondence: [email protected] Pimenta, V.T.; Paneto, G.G.; dos Santos, A.R.; Ditchfield, A.D.; Abstract: Faunal mortality from roadkill has a negative impact on global biodiversity, and bats are Bergallo, H.G.; Banhos, A. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Neoichnology of Bats: Morphological, Ecological, and Phylogenetic Influences on Terrestrial Behavior and Trackmaking Ability Within the Chiroptera
NEOICHNOLOGY OF BATS: MORPHOLOGICAL, ECOLOGICAL, AND PHYLOGENETIC INFLUENCES ON TERRESTRIAL BEHAVIOR AND TRACKMAKING ABILITY WITHIN THE CHIROPTERA BY MATTHEW FRAZER JONES Submitted to the graduate degree program in Geology and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Master of Science. Advisory Committee: ______________________________ Chairperson Stephen T. Hasiotis ______________________________ Co-chair David A. Burnham ______________________________ Robert M. Timm Date Defended: April 8, 2016 The Thesis Committee for MATTHEW FRAZER JONES certifies that this is the approved version of the following thesis: NEOICHNOLOGY OF BATS: MORPHOLOGICAL, ECOLOGICAL, AND PHYLOGENETIC INFLUENCES ON TERRESTRIAL BEHAVIOR AND TRACKMAKING ABILITY WITHIN THE CHIROPTERA ______________________________ Chairperson: Stephen T. Hasiotis ______________________________ Co-chairperson: David A. Burnham Date Approved: April 8, 2016 ii ABSTRACT Among living mammals, bats (Chiroptera) are second only to rodents in total number of species with over 1100 currently known. Extant bat species occupy many trophic niches and feeding habits, including frugivores (fruit eaters), insectivores (insect eaters), nectarivores (nectar and pollen-eaters), carnivores (predators of small terrestrial vertebrates), piscivores (fish eaters), sanguinivores (blood eaters), and omnivores (eat animals and plant material). Modern bats also demonstrate a wide range of terrestrial abilities while feeding, including: (1) those that primarily feed at or near ground level, such as the common vampire bat (Desmodus rotundus) and the New Zealand short-tailed bat (Mystacina tuberculata); (2) those rarely observed to feed from or otherwise spend time on the ground; and (3) many intermediate forms that demonstrate terrestrial competency without an obvious ecological basis. The variation in chiropteran terrestrial ability has been hypothesized to be constrained by the morphology of the pelvis and hindlimbs into what are termed types 1, 2, and 3 bats. -

Paleocene Mammalian Faunas of the Bison Basin in South-Central Wyoming
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 131, NUMBER 6 Cftarlesi ©, anb JHarp ^aux SKHalcott 3Res!earcf) Jf unb PALEOCENE MAMMALIAN FAUNAS OF THE BISON BASIN IN SOUTH-CENTRAL WYOMING (With 16 Plates) By C. LEWIS GAZIN Curator, Division of Vertebrate Paleontology United States National Museum Smithsonian Institution (Publication 4229) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION FEBRUARY 28, 1956 THE LORD BALTIMORE PRESS, INC. BALTIMORE, MD., U. S. A. CONTENTS Page Introduction i Acknowledgments 2 History of investigation 2 Occurrence and preservation of material 3 The Bison basin faunas 4 Environment and relationships between the Bison basin faunas 7 Age and correlation of the faunas lo Systematic description of vertebrate remains I2 Reptilia 12 Sauria 12 Anguidae 12 Mammalia 12 Multitubcrculata 12 Ptilodontidae 12 Marsupialia 14 Didelphidae 14 Insectivora 15 Leptictidae 15 Pantolestidae 17 Primates 19 Plesiadapidae 19 Carnivora 25 Arctocyonidae 25 Mesonychidae 35 Miacidae 35 Condylarthra 36 Hyopsodontidae 36 Phenacodontidae 42 Pantodonta 47 Coryphodontidae 47 References 51 Explanation of plates 54 ILLUSTRATIONS Plates (All plates following page 58.) 1. Mullituberculates and insectivores from the Bison basin Paleocene. 2. Primates and marsupials from the Bison basin Paleocene. 3. Pronothodcctes from the Bison basin Paleocene. 4. Plcsiadapis from the Bison basin Paleocene. 5. Triccntcs and Chriacus from the Bison basin Paleocene. 6. Thryptacodon from the Bison basin Paleocene. 7. Clacnodon from the Bison basin Paleocene. 8. Litomylus and Protoselcnc? from the Bison basin Paleocene. 9. Haplaletes and Gidleyina from the Bison basin Paleocene. 10. Phcnacodusl from the Bison basin Paleocene. 11. Condylarths and Titanoidcs from the Bison basin Paleocene. 12. Caenolambda from the Bison basin Paleocene. -
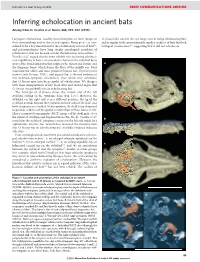
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

Mammalian Species 117
MAMMALIANSPECIES No. 117, pp. 14, 5 figs. Rhynchocyon chrysopygus. BY Galen B. Rathbun Published 8 June 1979 by the American Society of Mammalogists Rhynchocyon Peters, 1847 cyon chrysopygus apparently does not occur in the gallery forests of the Tana River, in the ground-water forest at Witu, or in the Rhynchocyon Peters, 1W7:36, type species Rhynchocyon cirnei dry bushlands between the Galana and Tana rivers. This ele- Peters by monotypy. phant-shrew's habitat is being cleared for exotic forest plantations Rhir~onaxThomas, 1918:370, type species Rh~nchoc~onchr~so- and agriculture all along the coast, resulting in a discontinuous pygus Gunther. and reduced distribution. It will re-occupy fallow agricultural land that is allowed to become overgrown with dense bush (Rathbun, CONTEXT AND CONTENT. Order Macroscelidea, Fam- ily Macroscelididae, Subfamily Rhynch~c~oninae.Corbet and unpublished data)' Hanks (1968) recognized three allopatric species of Rhynchocyon FOSSIL RECORD. As far as is known, the Macrosceli- (figure 1) for which they wrote the following key: didae have always been endemic to Africa (Patterson, 1%5). But- ler and Hopwood (1957) described Rhynchocyon clarki from the 1 Rump straw-colored, contrasting sharply with surround- early Miocene beds of Songhor, Kenya (near Lake Victoria). Ad- ing rufous pelage ----------.-------.-------R. chrysopygus ditional Miocene material from Rusinga Island, Kenya, has been Rump not straw-colored ---------.------.-------------------2 referred to this extinct form (Patterson, 1%5), which was smaller 2(1) Rump and posterior half of back with a pattern of dark than the extant species of Rhynchocyon. R. clarki contributes lines or spots on a yellowish-brown or rufous ground; significantly to the forest related fossil mammal fauna from Ru- top of head without a rufous tinge ._._._....._._R. -

Bird and Bat Conservation Strategy
Searchlight Wind Energy Project FEIS Appendix B 18B Appendix B-4: Bird and Bat Conservation Strategy Page | B Searchlight Bird and Bat Conservation Strategy Searchlight Wind Energy Project Bird and Bat Conservation Strategy Prepared for: Duke Energy Renewables 550 South Tryon Street Charlotte, North Carolina 28202 Prepared by: Tetra Tech EC, Inc. 1750 SW Harbor Way, Suite 400 Portland, OR 97201 November 2012 Searchlight BBCS i October 2012 Searchlight Bird and Bat Conservation Strategy TABLE OF CONTENTS 1.0 INTRODUCTION ............................................................................................................ 6 1.1 Duke Energy Renewables‘ Corporate Policy .......................................................... 6 1.2 Statement of Purpose ............................................................................................ 6 2.0 BACKGROUND AND DESCRIPTION OF THE PROJECT ............................................. 6 3.0 PROJECT-SPECIFIC REGULATORY REQUIREMENTS .............................................11 3.1 Potential Endangered Species Act-Listed Wildlife Species ...................................11 3.2 Migratory Bird Treaty Act ......................................................................................11 3.3 Bald and Golden Eagle Protection Act ..................................................................12 3.4 Nevada State Codes .............................................................................................12 4.0 DECISION FRAMEWORK .............................................................................................12 -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Lasiurus Egregius (Peters, 1870) (Chiroptera, Vespertilionidae) in Paraná State, Southern Brazil
15 6 NOTES ON GEOGRAPHIC DISTRIBUTION Check List 15 (6): 1099–1105 https://doi.org/10.15560/15.6.1099 First record of Lasiurus egregius (Peters, 1870) (Chiroptera, Vespertilionidae) in Paraná state, southern Brazil Fernando Carvalho1, 2, Daniela Aparecida Savariz Bôlla3, Karolaine Porto Supi1, Luana da Silva Biz1, 2, Beatriz Fernandes Lima Luciano1, 2, 4, Jairo José Zocche2, 4 1 Universidade do Extremo Sul Catarinense, Laboratório de Zoologia e Ecologia de Vertebrados, Av. Universitária n.1105, Bairro Universitário, Criciúma, SC, CEP: 80806-000, Brazil. 2 Universidade do Extremo Sul Catarinense, Programa de Pós-Graduação em Ciências Ambientais, Av. Universitária n.1105, Bairro Universitário, Criciúma, SC, CEP: 80806-000, Brazil. 3 National Institute for Amazonian Research (INPA), Post Graduation Program in Ecology, Av. André Araújo, n.2936, Bairro Petrópolis, Manaus, AM, CEP: 69.067-375, Brazil. 4 Universidade do Extremo Sul Catarinense, Laboratório de Ecologia de Paisagem e de Vertebrados Av. Universitária n.1105, Bairro Universitário, Criciúma, SC, CEP: 80806-000, Brazil. Corresponding author: Fernando Carvalho e-mail: [email protected] Abstract Lasiurus egregius (Peters, 1870) is an insectivorous bat species known from Central and South America. This species has few confirmed records throughout its distribution. Here we report the first record of L. egregius from the northern coast of Paraná state, southern Brazil. We captured a female individual of L. egregius using an ultrathin mist-net installed over a river knee, at Salto Morato Natural Reserve, municipality of Guaraqueçaba. This is the fourteenth locality with confirmed occurrence of L. egregius, being eight of them in Brazil. The knowledge on the bat fauna in Paraná has been increasing in recent decades, mainly due to the new studies in coast areas of this state. -

17 Roberts Road, Eastern Creek
17 ROBERTS ROAD, EASTERN CREEK SSD-10330 Proposed Data Centre Biodiversity Development Assessment Report Prepared for: Canberra Data Centres Pty Ltd PO Box 304 JERRABOMBERRA NSW 2619 SLR Ref: 610.18883-R05 Version No: -v2.0 November 2019 Canberra Data Centres Pty Ltd SLR Ref No: 610.18883-R05-v2.0-Eastern Creek BDAR-20191111.docx 17 Roberts Road, Eastern Creek November 2019 SSD-10330 Proposed Data Centre Biodiversity Development Assessment Report PREPARED BY SLR Consulting Australia Pty Ltd ABN 29 001 584 612 10 Kings Road New Lambton NSW 2305 Australia (PO Box 447 New Lambton NSW 2305 Australia) T: +61 2 4037 3200 E: [email protected] www.slrconsulting.com BASIS OF REPORT This report has been prepared by SLR Consulting Australia Pty Ltd (SLR) with all reasonable skill, care and diligence, and taking account of the timescale and resources allocated to it by agreement with Canberra Data Centres Pty Ltd (the Client). Information reported herein is based on the interpretation of data collected, which has been accepted in good faith as being accurate and valid. This report is for the exclusive use of the Client. No warranties or guarantees are expressed or should be inferred by any third parties. This report may not be relied upon by other parties without written consent from SLR. SLR disclaims any responsibility to the Client and others in respect of any matters outside the agreed scope of the work. DOCUMENT CONTROL Reference Date Prepared Checked Authorised 610.18883-R05-v2.0 11 November 2019 Fiona Iolini and David Martin Jeremy