Myzopodidae: Chiroptera) from Western Madagascar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity and Abundance of Roadkilled Bats in the Brazilian Atlantic Forest
diversity Article Diversity and Abundance of Roadkilled Bats in the Brazilian Atlantic Forest Lucas Damásio 1,2 , Laís Amorim Ferreira 3, Vinícius Teixeira Pimenta 3, Greiciane Gaburro Paneto 4, Alexandre Rosa dos Santos 5, Albert David Ditchfield 3,6, Helena Godoy Bergallo 7 and Aureo Banhos 1,3,* 1 Centro de Ciências Exatas, Naturais e da Saúde, Departamento de Biologia, Universidade Federal do Espírito Santo, Alto Universitário, s/nº, Guararema, Alegre 29500-000, ES, Brazil; [email protected] 2 Programa de Pós-Graduação em Ecologia, Instituto de Ciências Biológicas, Campus Darcy Ribeiro, Universidade de Brasília, Brasília 70910-900, DF, Brazil 3 Programa de Pós-Graduação em Ciências Biológicas (Biologia Animal), Universidade Federal do Espírito Santo, Av. Fernando Ferrari, 514, Prédio Bárbara Weinberg, Vitória 29075-910, ES, Brazil; [email protected] (L.A.F.); [email protected] (V.T.P.); [email protected] (A.D.D.) 4 Centro de Ciências Exatas, Naturais e da Saúde, Departamento de Farmácia e Nutrição, Universidade Federal do Espírito Santo, Alto Universitário, s/nº, Guararema, Alegre 29500-000, ES, Brazil; [email protected] 5 Centro de Ciências Agrárias e Engenharias, Departamento de Engenharia Rural, Universidade Federal do Espírito Santo, Alto Universitário, s/nº, Guararema, Alegre 29500-000, ES, Brazil; [email protected] 6 Centro de Ciências Humanas e Naturais, Departamento de Ciências Biológicas, Universidade Federal do Espírito Santo, Av. Fernando Ferrari, 514, Vitória 29075-910, ES, Brazil 7 Departamento de Ecologia, Instituto de Biologia Roberto Alcântara Gomes, Universidade do Estado do Rio de Janeiro, Rua São Francisco Xavier 524, Maracanã, Rio de Janeiro 20550-900, RJ, Brazil; [email protected] Citation: Damásio, L.; Ferreira, L.A.; * Correspondence: [email protected] Pimenta, V.T.; Paneto, G.G.; dos Santos, A.R.; Ditchfield, A.D.; Abstract: Faunal mortality from roadkill has a negative impact on global biodiversity, and bats are Bergallo, H.G.; Banhos, A. -

Special Publications Museum of Texas Tech University Number 63 18 September 2014
Special Publications Museum of Texas Tech University Number 63 18 September 2014 List of Recent Land Mammals of Mexico, 2014 José Ramírez-Pulido, Noé González-Ruiz, Alfred L. Gardner, and Joaquín Arroyo-Cabrales.0 Front cover: Image of the cover of Nova Plantarvm, Animalivm et Mineralivm Mexicanorvm Historia, by Francisci Hernández et al. (1651), which included the first list of the mammals found in Mexico. Cover image courtesy of the John Carter Brown Library at Brown University. SPECIAL PUBLICATIONS Museum of Texas Tech University Number 63 List of Recent Land Mammals of Mexico, 2014 JOSÉ RAMÍREZ-PULIDO, NOÉ GONZÁLEZ-RUIZ, ALFRED L. GARDNER, AND JOAQUÍN ARROYO-CABRALES Layout and Design: Lisa Bradley Cover Design: Image courtesy of the John Carter Brown Library at Brown University Production Editor: Lisa Bradley Copyright 2014, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (nsrl.ttu.edu). The authors and the Museum of Texas Tech University hereby grant permission to interested parties to download or print this publication for personal or educational (not for profit) use. Re-publication of any part of this paper in other works is not permitted without prior written permission of the Museum of Texas Tech University. This book was set in Times New Roman and printed on acid-free paper that meets the guidelines for per- manence and durability of the Committee on Production Guidelines for Book Longevity of the Council on Library Resources. Printed: 18 September 2014 Library of Congress Cataloging-in-Publication Data Special Publications of the Museum of Texas Tech University, Number 63 Series Editor: Robert J. -

Intestinal Helminths of the White Sucker, Catostomus Commersoni (Lacepede), in SE Wisconsin
OF WASHINGTON, VOLUME 41, NUMBER 1, JANUARY 1974 81 Intestinal Helminths of the White Sucker, Catostomus commersoni (Lacepede), in SE Wisconsin OMAR M. AMIN Science Division, University of Wisconsin-Parkside, Kenosha 53140 ABSTRACT: Five species of helminths were recovered from the intestine of the white sucker, Catostomus commersoni (Lacepede), in southeastern Wisconsin. Hosts were seined in five sites in both the Root River (Milwaukee and Racine counties; autumn 1971) and the Pike River (Racine and Kenosha counties; autumn 1972). The helminths are Triganodistomum attenuatum Mueller and Van Cleave, 1932 (Trem- atoda: Lissorchiidae), new locality record; Eiacetabulum macrocephalum McCrae, 1962 (Cestoda: Caryophyllaeidae), new state record; Biacetabulum biloculoides Mackiewicz and McCrae, 1965 (Cestoda: Caryophyllaeidae), new state record; Acanthocephahis dims (Van Cleave, 1931) Van Cleave and Townsend, 1936, new host and state record; Dorylaimtis sp. (?) Dujardin, 1845 (Nematoda: Dory- laimidae), a nonparasitie nematode reported for the first time in this fish. Distribution, structural obser- vations, and host-parasite relationships of the above species are discussed. Previous reports of fish parasites in Wis- upon recovery were placed in 70% alcohol. consin were confined to the geographical Hosts were classed in one of three size classes northeast and west (Pearse, 1924a), north according to their total length, 5-9.9, 10-14.9, (Bangham, 1946), northwest (Fischthal, 1947, and 15-37 cm. 1950, 1952), and east (Anthony, 1963). These Trematodes and cestodes were stained in reports dealt only with host records. Literature Semichon's carmine, cleared in xylene, and on the ecology or host-parasite relationship of whole-mounted in Canada balsam. Acantho- fish parasites in Wisconsin is relatively scarce cephalans were fixed in Bouin's fluid, stained (Marshall and Gilbert, 1905; Pearse, 1924b; in Harris' hematoxylin, cleared in beechwood Cross, 1938). -

(Nematoda: Thelazioidea) in the Blue Sucker, Cycleptus Elongatus (Lesueur, 1817), from Illinois
Transactions of the Illinois State Academy of Science received 12/10/01 (2002), Volume 95, #2, pp. 107 - 109 accepted 2/22/02 First Record of Rhabdochona cascadilla Wigdor, 1918 (Nematoda: Thelazioidea) in the Blue Sucker, Cycleptus elongatus (Lesueur, 1817), from Illinois William G. Dyer and William J. Poly Department of Zoology Southern Illinois University Carbondale, Illinois 62901-6501 ABSTRACT Rhabdochona cascadilla was detected in the intestine of Cycleptus elongatus from the Mississippi River in Randolph County, Illinois. This constitutes the first record of this rhabdochonid nematode in the blue sucker and the only internal helminth for this host. INTRODUCTION Nematodes of the genus Rhabdochona Railliet, 1916 (Thelazioidea, Rhabdochonidae) are cosmopolitan in distribution as intestinal parasites of freshwater fishes (Moravec and Coy Otero, 1987; Moravec, 1994). Approximately 96 nominal species have been recognized of which 19 have been reported from North and South America (see Sanchez-Alvarez et al., 1998). Of rhabdochonid nematodes reported from North America, Rhabdochona cascadilla Wigdor, 1918 has been recorded in 13 families, 30 genera, and 53 species of freshwater fishes across Canada and the United States (Hoffman, 1999). Identification of Rhabdochona species is difficult as many of them have been inadequately or erroneously described, and as pointed out by Sanchez-Alvarez et al. (1998), a detailed taxonomic revision of all putative species in this group warrants initiation. MATERIALS AND METHODS A single adult female blue sucker (557 mm standard length) was collected incidentally by electrofishing on 24 October 1996 from the Mississippi River just below the mouth of the Kaskaskia River, Randolph County, Illinois and was transported alive to the laboratory. -

Ecosystem Profile Madagascar and Indian
ECOSYSTEM PROFILE MADAGASCAR AND INDIAN OCEAN ISLANDS FINAL VERSION DECEMBER 2014 This version of the Ecosystem Profile, based on the draft approved by the Donor Council of CEPF was finalized in December 2014 to include clearer maps and correct minor errors in Chapter 12 and Annexes Page i Prepared by: Conservation International - Madagascar Under the supervision of: Pierre Carret (CEPF) With technical support from: Moore Center for Science and Oceans - Conservation International Missouri Botanical Garden And support from the Regional Advisory Committee Léon Rajaobelina, Conservation International - Madagascar Richard Hughes, WWF – Western Indian Ocean Edmond Roger, Université d‘Antananarivo, Département de Biologie et Ecologie Végétales Christopher Holmes, WCS – Wildlife Conservation Society Steve Goodman, Vahatra Will Turner, Moore Center for Science and Oceans, Conservation International Ali Mohamed Soilihi, Point focal du FEM, Comores Xavier Luc Duval, Point focal du FEM, Maurice Maurice Loustau-Lalanne, Point focal du FEM, Seychelles Edmée Ralalaharisoa, Point focal du FEM, Madagascar Vikash Tatayah, Mauritian Wildlife Foundation Nirmal Jivan Shah, Nature Seychelles Andry Ralamboson Andriamanga, Alliance Voahary Gasy Idaroussi Hamadi, CNDD- Comores Luc Gigord - Conservatoire botanique du Mascarin, Réunion Claude-Anne Gauthier, Muséum National d‘Histoire Naturelle, Paris Jean-Paul Gaudechoux, Commission de l‘Océan Indien Drafted by the Ecosystem Profiling Team: Pierre Carret (CEPF) Harison Rabarison, Nirhy Rabibisoa, Setra Andriamanaitra, -

African Bat Conservation News
Volume 35 African Bat Conservation News August 2014 ISSN 1812-1268 © ECJ Seamark, 2009 (AfricanBats) Above: A male Cape Serotine Bat (Neoromicia capensis) caught in the Chitabi area, Okavango Delta, Botswana. Inside this issue: Research and Conservation Activities Presence of paramyxo and coronaviruses in Limpopo caves, South Africa 2 Observations, Discussions and Updates Recent changes in African Bat Taxonomy (2013-2014). Part II 3 Voucher specimen details for Bakwo Fils et al. (2014) 4 African Chiroptera Report 2014 4 Scientific contributions Documented record of Triaenops menamena (Family Hipposideridae) in the Central Highlands of 6 Madagascar Download and subscribe to African Bat Conservation News published by AfricanBats at: www.africanbats.org The views and opinions expressed in articles are no necessarily those of the editor or publisher. Articles and news items appearing in African Bat Conservation News may be reprinted, provided the author’s and newsletter refer- ence are given. African Bat Conservation News August 2014 vol. 35 2 ISSN 1812-1268 Inside this issue Continued: Recent Literature Conferences 7 Published Books / Reports 7 Papers 7 Notice Board Conferences 13 Call for Contributions 13 Research and Conservation Activities Presence of paramyxo- and coronaviruses in Limpopo caves, South Africa By Carmen Fensham Department of Microbiology and Plant Pathology, Faculty of Natural and Agricultural Sciences, University of Pretoria, 0001, Republic of South Africa. Correspondence: Prof. Wanda Markotter: [email protected] Carmen Fensham is a honours excrement are excised and used to isolate any viral RNA that student in the research group of may be present. The identity of the RNA is then determined Prof. -

Digenean Metacercaria (Trematoda, Digenea, Lepocreadiidae) Parasitizing “Coelenterates” (Cnidaria, Scyphozoa and Ctenophora) from Southeastern Brazil
BRAZILIAN JOURNAL OF OCEANOGRAPHY, 53(1/2):39-45, 2005 DIGENEAN METACERCARIA (TREMATODA, DIGENEA, LEPOCREADIIDAE) PARASITIZING “COELENTERATES” (CNIDARIA, SCYPHOZOA AND CTENOPHORA) FROM SOUTHEASTERN BRAZIL André Carrara Morandini1; Sergio Roberto Martorelli2; Antonio Carlos Marques1 & Fábio Lang da Silveira1 1Instituto de Biociências da Universidade de São Paulo Departamento de Zoologia (Caixa Postal 11461, 05422-970, São Paulo, SP, Brazil) 2Centro de Estudios Parasitologicos y Vectores (CEPAVE) (2 Nro. 584 (1900) La Plata, Argentina) e-mails: [email protected], [email protected], [email protected], [email protected] A B S T R A C T Metacercaria specimens of the genus Opechona (Trematoda: Digenea: Lepocreadiidae) are described parasitizing “coelenterates” (scyphomedusae and ctenophores) from Southeastern Brazil (São Paulo state). The worms are compared to other Opechona species occurring on the Brazilian coast, but no association has been made because only adult forms of these species have been described. Suppositions as to the possible transference of the parasites are made. R E S U M O Exemplares de metacercárias do gênero Opechona (Trematoda: Digenea: Lepocreadiidae) são descritos parasitando “celenterados” (cifomedusas e ctenóforos) no sudeste do Brasil (estado de São Paulo). Os vermes foram comparados a outras espécies de Opechona ocorrentes no litoral brasileiro, porém nenhuma associação foi realizada devido às demais espécies terem sido descritas a partir de exemplares adultos. São apresentadas suposições sobre as possíveis formas -

Shortnose Sucker (Chasmistes Brevirostris) 5-Year Review Summary and Evaluation
Shortnose Sucker (Chasmistes brevirostris) 5-Year Review Summary and Evaluation U.S. Fish and Wildlife Service Klamath Falls Fish and Wildlife Office Klamath Falls, Oregon July 2007 5-YEAR REVIEW Shortnose Sucker (Chasmistes brevirostris) TABLE OF CONTENTS 1.0 GENERAL INFORMATION.......................................................................................... 3 1.1. Reviewers............................................................................................................................ 3 1.2. Methodology used to complete the review....................................................................... 3 1.3. Background ........................................................................................................................ 3 2.0 REVIEW ANALYSIS....................................................................................................... 4 2.1. Application of the 1996 Distinct Populations Segment (DPS) policy ............................ 4 2.2. Biology and Habitat ........................................................................................................... 5 2.3. Recovery Criteria............................................................................................................. 13 2.4. Five-Factor Analysis ........................................................................................................ 16 2.5. Synthesis............................................................................................................................ 30 3.0 RESULTS ....................................................................................................................... -

AP ART HISTORY--Unit 5 Study Guide (Non Western Art Or Art Beyond the European Tradition) Ms
AP ART HISTORY--Unit 5 Study Guide (Non Western Art or Art Beyond the European Tradition) Ms. Kraft BUDDHISM Important Chronology in the Study of Buddhism Gautama Sakyamuni born ca. 567 BCE Buddhism germinates in the Ganges Valley ca. 487-275 BCE Reign of King Ashoka ca. 272-232 BCE First Indian Buddha image ca. 1-200 CE First known Chinese Buddha sculpture 338 CE First known So. China Buddha image 437 CE The Four Noble Truthsi 1. Life means suffering. Life is full of suffering, full of sickness and unhappiness. Although there are passing pleasures, they vanish in time. 2. The origin of suffering is attachment. People suffer for one simple reason: they desire things. It is greed and self-centeredness that bring about suffering. Desire is never satisfied. 3. The cessation of suffering is attainable. It is possible to end suffering if one is aware of his or her own desires and puts and end to them. This awareness will open the door to lasting peace. 4. The path to the cessation of suffering. By changing one’s thinking and behavior, a new awakening can be reached by following the Eightfold Path. Holy Eightfold Pathii The principles are • Right Understanding • Right Intention • Right Speech • Right Action • Right Livelihood • Right Effort • Right Awareness • Right Concentration Following the Eightfold Path will lead to Nirvana or salvation from the cycle of rebirth. Iconography of the Buddhaiii The image of the Buddha is distinguished in various different ways. The Buddha is usually shown in a stylized pose or asana. Also important are the 32 lakshanas or special bodily features or birthmarks. -

Major Geographic Qualities of South Asia
South Asia Chapter 8, Part 1: pages 402 - 417 Teacher Notes DEFINING THE REALM I. Major geographic qualities of South Asia - Well defined physiography – bordered by mountains, deserts, oceans - Rivers – The Ganges supports most of population for over 10,000 years - World’s 2nd largest population cluster on 3% land mass - Current birth rate will make it 1st in population in a decade - Low income economies, inadequate nutrition and poor health - British imprint is strong – see borders and culture - Monsoons, this cycle sustains life, to alter it would = disaster - Strong cultural regionalism, invading armies and cultures diversified the realm - Hindu, Buddhists, Islam – strong roots in region - India is biggest power in realm, but have trouble with neighbors - Kashmir – dangerous source of friction between 2 nuclear powers II. Defining the Realm Divided along arbitrary lines drawn by England Division occurred in 1947 and many lives were lost This region includes: Pakistan (East & West), India, Bangladesh (1971), Sri Lanka, Maldives Islands Language – English is lingua franca III. Physiographic Regions of South Asia After Russia left Afghanistan Islamic revivalism entered the region Enormous range of ecologies and environment – Himalayas, desert and tropics 1) Monsoons – annual rains that are critical to life in that part of the world 1 2) Regions: A. Northern Highlands – Himalayas, Bhutan, Afghanistan B. River Lowlands – Indus Valley in Pakistan, Ganges Valley in India, Bangladesh C. Southern Plateaus – throughout much of India, a rich -
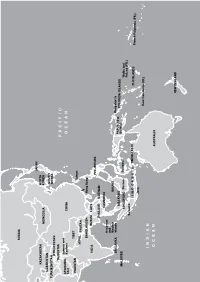
Asia and Oceania Nicole Girard, Irwin Loy, Marusca Perazzi, Jacqui Zalcberg the Country
ARCTIC OCEAN RUSSIA JAPAN KAZAKHSTAN NORTH MONGOLIA KOREA UZBEKISTAN SOUTH TURKMENISTAN KOREA KYRGYZSTAN TAJIKISTAN PACIFIC Jammu and AFGHANIS- Kashmir CHINA TAN OCEAN PAKISTAN TIBET Taiwan NEPAL BHUTAN BANGLADESH Hong Kong INDIA BURMA LAOS PHILIPPINES THAILAND VIETNAM CAMBODIA Andaman and Nicobar BRUNEI SRI LANKA Islands Bougainville MALAYSIA PAPUA NEW SOLOMON ISLANDS MALDIVES GUINEA SINGAPORE Borneo Sulawesi Wallis and Futuna (FR.) Sumatra INDONESIA TIMOR-LESTE FIJI ISLANDS French Polynesia (FR.) Java New Caledonia (FR.) INDIAN OCEAN AUSTRALIA NEW ZEALAND Asia and Oceania Nicole Girard, Irwin Loy, Marusca Perazzi, Jacqui Zalcberg the country. However, this doctrine is opposed by nationalist groups, who interpret it as an attack on ethnic Kazakh identity, language and Central culture. Language policy is part of this debate. The Asia government has a long-term strategy to gradually increase the use of Kazakh language at the expense Matthew Naumann of Russian, the other official language, particularly in public settings. While use of Kazakh is steadily entral Asia was more peaceful in 2011, increasing in the public sector, Russian is still with no repeats of the large-scale widely used by Russians, other ethnic minorities C violence that occurred in Kyrgyzstan and many urban Kazakhs. Ninety-four per cent during the previous year. Nevertheless, minor- of the population speak Russian, while only 64 ity groups in the region continue to face various per cent speak Kazakh. In September, the Chair forms of discrimination. In Kazakhstan, new of the Kazakhstan Association of Teachers at laws have been introduced restricting the rights Russian-language Schools reportedly stated in of religious minorities. Kyrgyzstan has seen a a roundtable discussion that now 56 per cent continuation of harassment of ethnic Uzbeks in of schoolchildren study in Kazakh, 33 per cent the south of the country, and pressure over land in Russian, and the rest in smaller minority owned by minority ethnic groups. -

Lasiurus Egregius (Peters, 1870) (Chiroptera, Vespertilionidae) in Paraná State, Southern Brazil
15 6 NOTES ON GEOGRAPHIC DISTRIBUTION Check List 15 (6): 1099–1105 https://doi.org/10.15560/15.6.1099 First record of Lasiurus egregius (Peters, 1870) (Chiroptera, Vespertilionidae) in Paraná state, southern Brazil Fernando Carvalho1, 2, Daniela Aparecida Savariz Bôlla3, Karolaine Porto Supi1, Luana da Silva Biz1, 2, Beatriz Fernandes Lima Luciano1, 2, 4, Jairo José Zocche2, 4 1 Universidade do Extremo Sul Catarinense, Laboratório de Zoologia e Ecologia de Vertebrados, Av. Universitária n.1105, Bairro Universitário, Criciúma, SC, CEP: 80806-000, Brazil. 2 Universidade do Extremo Sul Catarinense, Programa de Pós-Graduação em Ciências Ambientais, Av. Universitária n.1105, Bairro Universitário, Criciúma, SC, CEP: 80806-000, Brazil. 3 National Institute for Amazonian Research (INPA), Post Graduation Program in Ecology, Av. André Araújo, n.2936, Bairro Petrópolis, Manaus, AM, CEP: 69.067-375, Brazil. 4 Universidade do Extremo Sul Catarinense, Laboratório de Ecologia de Paisagem e de Vertebrados Av. Universitária n.1105, Bairro Universitário, Criciúma, SC, CEP: 80806-000, Brazil. Corresponding author: Fernando Carvalho e-mail: [email protected] Abstract Lasiurus egregius (Peters, 1870) is an insectivorous bat species known from Central and South America. This species has few confirmed records throughout its distribution. Here we report the first record of L. egregius from the northern coast of Paraná state, southern Brazil. We captured a female individual of L. egregius using an ultrathin mist-net installed over a river knee, at Salto Morato Natural Reserve, municipality of Guaraqueçaba. This is the fourteenth locality with confirmed occurrence of L. egregius, being eight of them in Brazil. The knowledge on the bat fauna in Paraná has been increasing in recent decades, mainly due to the new studies in coast areas of this state.