2 Icaronycteris Index
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Confirmed Breeding Record of Plume-Toed Swiftlet Collocalia Affinis in Singapore
BirdingASIA 31 (2019)(2019): 85–87 85 BREEDING RECORD First confirmed breeding record of Plume-toed Swiftlet Collocalia affinis in Singapore ALFRED CHIA, DING LI YONG, KIM CHUAH LIM, MOVIN NYANASENGERAN, YONG CHEE KEITA SIN, KIM KEANG LIM & SENG BENG YEO Historically the tiny Collocalia swiftlets resident In the meantime, the results of a detailed in both the lowlands and higher altitudes of analysis of the taxonomy of Collocalia swiftlets in Peninsular Malaysia were designated Glossy the Indo-Pacific region (Rheindt et al. 2017) led to Swiftlet C. esculenta cyanoptila (Wells 1999). the proposal of radical changes to the established However, the species was rare on Singapore taxonomy; this was a big challenge due to the mainland, with fewer than 10 records per year, all morphological uniformity of these taxa. Rheindt apparently in the period November to February, et al. (2017) studied the evolutionary history of the which is the non-breeding season in Malaysia complex, combining new biometric measurements (Wells 1999). In recent times the frequency of and plumage assessment of museum specimens records in Singapore has increased, including a with novel as well as previously published group of up to seven birds at Bukit Batok Nature molecular data, with a total of 809 individuals Park in January 2005, as well as regular records representative of 32 taxa being assessed. The thereafter in and at the periphery of the Central authors propose changing the classification of Nature Reserves, and its status was revised to white-bellied swiftlets, for which just two species ‘uncommon’ (Yong et al. 2017, Lim 2018). -

Morphometrical Variations of Malaysian Hipposideros Species
Malaysian Journal of Mathematical Sciences 6(1): 47-57 (2012) Morphometrical Variations of Malaysian Hipposideros Species Siti Nurlydia Sazali, Charlie J. Laman and M.T. Abdullah Department of Zoology, Faculty of Resource Science and Technology, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia E-mail: [email protected] ABSTRACT A study on the morphometrical variations among four Malaysian Hipposideros species was conducted using voucher specimens deposited in Universiti Malaysia Sarawak (UNIMAS) Zoological Museum and the Department of Widlife and National Park (DWNP) Kuala Lumpur. Twenty two individuals from four species of Hipposideros ater , H. bicolor , H. cineraceus and H. dyacorum were morphologically measured, in which a total of 27 linear parameters of body, skull and dentals of each were appropriately recorded. The statistical data were later subjected to discriminant function analysis (DFA) and canonical variate analysis (CVA) using SPSS version 15.0 and unweighted pair-group method average (UPGMA) cluster analysis using Minitab version 14.4. The highest character loadings observed in Function l, Function 2 and Function 3 were the forearm length (FA), the third digit second phalanx length (D3P2L) and the palatal length (PL) with standardised canonical discriminant function coefficient values of 21.910, 5.770 and 5.095, respectively. These three characters were identified as the best diagnostic features for discriminating these closely related species of Hipposideros . Hence, this morphometric approach could be a promising tool as an alternative to the molecular DNA analysis for identification of Chiroptera species. Keywords: Hipposideros , morphometric, discriminant function analysis cluster analysis, species identification. 1. INTRODUCTION Bats belong to the order Chiroptera and can be distinguished from all other mammals by their ability to fly, which is a result of the modification of their forelimbs into wings (Payne et al . -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Volume 41, 2000
BAT RESEARCH NEWS Volume 41 : No. 1 Spring 2000 I I BAT RESEARCH NEWS Volume 41: Numbers 1–4 2000 Original Issues Compiled by Dr. G. Roy Horst, Publisher and Managing Editor of Bat Research News, 2000. Copyright 2011 Bat Research News. All rights reserved. This material is protected by copyright and may not be reproduced, transmitted, posted on a Web site or a listserve, or disseminated in any form or by any means without prior written permission from the Publisher, Dr. Margaret A. Griffiths. The material is for individual use only. Bat Research News is ISSN # 0005-6227. BAT RESEARCH NEWS Volume41 Spring 2000 Numberl Contents Resolution on Rabies Exposure Merlin Tuttle and Thomas Griffiths o o o o eo o o o • o o o o o o o o o o o o o o o o 0 o o o o o o o o o o o 0 o o o 1 E - Mail Directory - 2000 Compiled by Roy Horst •••• 0 ...................... 0 ••••••••••••••••••••••• 2 ,t:.'. Recent Literature Compiled by :Margaret Griffiths . : ....••... •"r''• ..., .... >.•••••• , ••••• • ••< ...... 19 ,.!,..j,..,' ""o: ,II ,' f 'lf.,·,,- .,'b'l: ,~··.,., lfl!t • 0'( Titles Presented at the 7th Bat Researc:b Confei'ebee~;Moscow :i'\prill4-16~ '1999,., ..,, ~ .• , ' ' • I"',.., .. ' ""!' ,. Compiled by Roy Horst .. : .......... ~ ... ~· ....... : :· ,"'·~ .• ~:• .... ; •. ,·~ •.•, .. , ........ 22 ·.t.'t, J .,•• ~~ Letters to the Editor 26 I ••• 0 ••••• 0 •••••••••••• 0 ••••••• 0. 0. 0 0 ••••••• 0 •• 0. 0 •••••••• 0 ••••••••• 30 News . " Future Meetings, Conferences and Symposium ..................... ~ ..,•'.: .. ,. ·..; .... 31 Front Cover The illustration of Rhinolophus ferrumequinum on the front cover of this issue is by Philippe Penicaud . from his very handsome series of drawings representing the bats of France. -

BONNER ZOOLOGISCHE MONOGRAPHIEN, Nr
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zoologicalbulletin.de; www.biologiezentrum.at NEW WORLD NECTAR-FEEDING BATS: BIOLOGY, MORPHOLOGY AND CRANIOMETRIC APPROACH TO SYSTEMATICS by ERNST-HERMANN SOLMSEN BONNER ZOOLOGISCHE MONOGRAPHIEN, Nr. 44 1998 Herausgeber: ZOOLOGISCHES FORSCHUNGSINSTITUT UND MUSEUM ALEXANDER KOENIG BONN © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zoologicalbulletin.de; www.biologiezentrum.at BONNER ZOOLOGISCHE MONOGRAPHIEN Die Serie wird vom Zoologischen Forschungsinstitut und Museum Alexander Koenig herausgegeben und bringt Originalarbeiten, die für eine Unterbringung in den „Bonner zoologischen Beiträgen" zu lang sind und eine Veröffentlichung als Monographie rechtfertigen. Anfragen bezüglich der Vorlage von Manuskripten sind an die Schriftleitung zu richten; Bestellungen und Tauschangebote bitte an die Bibliothek des Instituts. This series of monographs, published by the Zoological Research Institute and Museum Alexander Koenig, has been established for original contributions too long for inclu- sion in „Bonner zoologische Beiträge". Correspondence concerning manuscripts for pubhcation should be addressed to the editor. Purchase orders and requests for exchange please address to the library of the institute. LTnstitut de Recherches Zoologiques et Museum Alexander Koenig a etabh cette serie de monographies pour pouvoir publier des travaux zoologiques trop longs pour etre inclus dans les „Bonner zoologische Beiträge". Toute correspondance concernante -
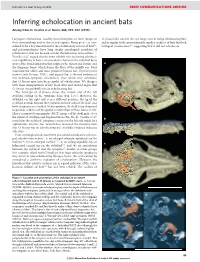
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

View Preprint
Does evolution of plumage patterns and of migratory behaviour in Apodini swifts (Aves: Apodiformes) follow distributional range shifts? Dieter Thomas Tietze, Martin Päckert, Michael Wink The Apodini swifts in the Old World serve as an example for a recent radiation on an intercontinental scale on the one hand. On the other hand they provide a model for the interplay of trait and distributional range evolution with speciation, extinction and trait s t transition rates on a low taxonomic level (23 extant taxa). Swifts are well adapted to a life n i mostly in the air and to long-distance movements. Their overall colouration is dull, but r P lighter feather patches of chin and rump stand out as visual signals. Only few Apodini taxa e r breed outside the tropics; they are the only species in the study group that migrate long P distances to wintering grounds in the tropics and subtropics. We reconstructed a dated molecular phylogeny including all species, numerous outgroups and fossil constraints. Several methods were used for historical biogeography and two models for the study of trait evolution. We finally correlated trait expression with geographic status. The differentiation of the Apodini took place in less than 9 Ma. Their ancestral range most likely comprised large parts of the Old-World tropics, although the majority of extant taxa breed in the Afrotropic and the closest relatives occur in the Indomalayan. The expression of all three investigated traits increased speciation rates and the traits were more likely lost than gained. Chin patches are found in almost all species, so that no association with phylogeny or range could be found. -

A Recent Bat Survey Reveals Bukit Barisan Selatan Landscape As A
A Recent Bat Survey Reveals Bukit Barisan Selatan Landscape as a Chiropteran Diversity Hotspot in Sumatra Author(s): Joe Chun-Chia Huang, Elly Lestari Jazdzyk, Meyner Nusalawo, Ibnu Maryanto, Maharadatunkamsi, Sigit Wiantoro, and Tigga Kingston Source: Acta Chiropterologica, 16(2):413-449. Published By: Museum and Institute of Zoology, Polish Academy of Sciences DOI: http://dx.doi.org/10.3161/150811014X687369 URL: http://www.bioone.org/doi/full/10.3161/150811014X687369 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Acta Chiropterologica, 16(2): 413–449, 2014 PL ISSN 1508-1109 © Museum and Institute of Zoology PAS doi: 10.3161/150811014X687369 A recent -

Carpe Noctem: the Importance of Bats As Bioindicators
Vol. 8: 93–115, 2009 ENDANGERED SPECIES RESEARCH Printed July 2009 doi: 10.3354/esr00182 Endang Species Res Published online April 8, 2009 Contribution to the Theme Section ‘Bats: status, threats and conservation successes’ OPENPEN REVIEW ACCESSCCESS Carpe noctem: the importance of bats as bioindicators Gareth Jones1,*, David S. Jacobs2, Thomas H. Kunz3, Michael R. Willig4, Paul A. Racey5 1School of Biological Sciences, University of Bristol, Woodland Road, Bristol BS8 1UG, UK 2Zoology Department, University of Cape Town, Rondesbosch 7701, Cape Town, South Africa 3Center for Ecology and Conservation Biology, Department of Biology, Boston University, Boston, Massachusetts 02215, USA 4Center for Environmental Sciences and Engineering and Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, Connecticut 06269-4210, USA 5School of Biological Sciences, University of Aberdeen, Tillydrone Avenue, Aberdeen AB24 2TN, UK ABSTRACT: The earth is now subject to climate change and habitat deterioration on unprecedented scales. Monitoring climate change and habitat loss alone is insufficient if we are to understand the effects of these factors on complex biological communities. It is therefore important to identify bioindicator taxa that show measurable responses to climate change and habitat loss and that reflect wider-scale impacts on the biota of interest. We argue that bats have enormous potential as bioindi- cators: they show taxonomic stability, trends in their populations can be monitored, short- and long- term effects on populations can be measured and they are distributed widely around the globe. Because insectivorous bats occupy high trophic levels, they are sensitive to accumulations of pesti- cides and other toxins, and changes in their abundance may reflect changes in populations of arthro- pod prey species. -

Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam
viruses Article Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam Satoru Arai 1, Fuka Kikuchi 1,2, Saw Bawm 3 , Nguyễn Trường Sơn 4,5, Kyaw San Lin 6, Vương Tân Tú 4,5, Keita Aoki 1,7, Kimiyuki Tsuchiya 8, Keiko Tanaka-Taya 1, Shigeru Morikawa 9, Kazunori Oishi 1 and Richard Yanagihara 10,* 1 Infectious Disease Surveillance Center, National Institute of Infectious Diseases, Tokyo 162-8640, Japan; [email protected] (S.A.); [email protected] (F.K.); [email protected] (K.A.); [email protected] (K.T.-T.); [email protected] (K.O.) 2 Department of Chemistry, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan 3 Department of Pharmacology and Parasitology, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; [email protected] 4 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi, Vietnam; [email protected] (N.T.S.); [email protected] (V.T.T.) 5 Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Hanoi, Vietnam 6 Department of Aquaculture and Aquatic Disease, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; [email protected] 7 Department of Liberal Arts, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan 8 Laboratory of Bioresources, Applied Biology Co., Ltd., Tokyo 107-0062, Japan; [email protected] 9 Department of Veterinary Science, National Institute of Infectious Diseases, Tokyo 162-8640, Japan; [email protected] 10 Pacific Center for Emerging Infectious Diseases Research, John A. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Echolocation and Foraging Behavior of the Lesser Bulldog Bat, Noctilio Albiventris : Preadaptations for Piscivory?
Behav Ecol Sociobiol (1998) 42: 305±319 Ó Springer-Verlag 1998 Elisabeth K. V. Kalko á Hans-Ulrich Schnitzler Ingrid Kaipf á Alan D. Grinnell Echolocation and foraging behavior of the lesser bulldog bat, Noctilio albiventris : preadaptations for piscivory? Received: 21 April 1997 / Accepted after revision: 12 January 1998 Abstract We studied variability in foraging behavior of can be interpreted as preadaptations favoring the evo- Noctilio albiventris (Chiroptera: Noctilionidae) in Costa lution of piscivory as seen in N. leporinus. Prominent Rica and Panama and related it to properties of its among these specializations are the CF components of echolocation behavior. N. albiventris searches for prey in the echolocation signals which allow detection and high (>20 cm) or low (<20 cm) search ¯ight, mostly evaluation of ¯uttering prey amidst clutter-echoes, high over water. It captures insects in mid-air (aerial cap- variability in foraging strategy and the associated tures) and from the water surface (pointed dip). We once echolocation behavior, as well as morphological spe- observed an individual dragging its feet through the cializations such as enlarged feet for capturing prey from water (directed random rake). In search ¯ight, N. al- the water surface. biventris emits groups of echolocation signals (duration 10±11 ms) containing mixed signals with constant-fre- Key words Bats á Echolocation á Foraging á quency (CF) and frequency-modulated (FM) compo- Evolution á Piscivory nents, or pure CF signals. Sometimes, mostly over land, it produces long FM signals (duration 15±21 ms). When N. albiventris approaches prey in a pointed dip or in aerial captures, pulse duration and pulse interval are Introduction reduced, the CF component is eliminated, and a termi- nal phase with short FM signals (duration 2 ms) at high The development of ¯ight and echolocation give bats repetition rates (150±170 Hz) is emitted.