Bsc Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract Multicomponent Reactions of Salicylaldehyde, Cyclic Ketones, and Arylamines Through Cooperative Enamine-Metal Lewis
ABSTRACT MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS by Ryan Gregory Sarkisian Multicomponent reactions (MCRs) are the most atom economic, highly selective, and convergent type of reaction. This allows for a reaction to have a wide scope and allows for maximization of the complexity of a product. Catalyzing these MCRs with asymmetric catalysis is a novel way to introduce stereocontrol into highly complex molecules with various functional groups. Asymmetric catalysis is considered the most efficient method for constructing highly functionalized optically active stereopure compounds. There are three pillars of asymmetric catalysis: biocatalysis, transition metal catalysis, and organocatalysis. This research focuses on two of these pillars, transition metal catalysis and organocatalysis, working cooperatively to catalyze this MCR. The focus is to educate or refresh the audience on the basic topics that make up the complexity of the MCRs being catalyzed by cooperative asymmetric catalysis. Ultimately to explore the cooperative catalysts used to synthesize both the racemic and asymmetric three fused ring products (9-((4-methoxyphenyl)amino)-2,3,4,4a,9,9a-hexahydro-1H-xanthen-4a-ol). MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS A THESIS Submitted to the Faculty of Miami University in partial fulfillment of the requirements for the degree of Master of Science Department of -

Catalytic Direct Asymmetric Michael Reactions
ORGANIC LETTERS 2001 Catalytic Direct Asymmetric Michael Vol. 3, No. 23 Reactions: Taming Naked Aldehyde 3737-3740 Donors Juan M. Betancort and Carlos F. Barbas III* The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 [email protected] Received September 5, 2001 ABSTRACT Direct catalytic enantio- and diastereoselective Michael addition reactions of unmodified aldehydes to nitro olefins using (S)-2-(morpholinomethyl)- pyrrolidine as a catalyst are described. The reactions proceed in good yield (up to 96%) in a highly syn-selective manner (up to 98:2) with enantioselectivities approaching 80%. The resulting γ-formyl nitro compounds are readily converted to chiral, nonracemic 3,4-disubstituted pyrrolidines. The Michael reaction is generally regarded as one of the Typically, carbon nucleophiles that contain an active most efficient carbon-carbon bond forming reactions, and methylene center such as malonic acid esters, â-keto esters, studies concerning this reaction have played an important nitroalkanes, etc. have been studied in the Michael reaction. role in the development of modern synthetic organic Carbonyl compounds, and ketones in particular, have gener- chemistry.1 As the demand for optically active compounds ally only been used as donors following their preactivation has soared in recent years, much progress has been made in by conversion into a more reactive species such as enol or the development of asymmetric variants of this reaction, enamine equivalents.5,6 In these cases, additional synthetic providing for the preparation of Michael adducts with high enantiomeric purity.2 Though remarkable advances have been (3) (a) Chataigner, I.; Gennari, C.; Ongeri, S.; Piarulli, U.; Ceccarelli, S. -

Study of Reactions of Two Mannich Bases Derived of 4’-Hydroxychalcones with Glutathione by RP‑TLC, RP‑HPLC and RP‑HPLC‑ESI‑MS Analysis
http://dx.doi.org/10.21577/0103-5053.20160260 J. Braz. Chem. Soc., Vol. 28, No. 6, 1048-1062, 2017. Printed in Brazil - ©2017 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00 Article Study of Reactions of Two Mannich Bases Derived of 4’-Hydroxychalcones with Glutathione by RP-TLC, RP-HPLC and RP-HPLC-ESI-MS Analysis Aline Bernardes,a,b Caridad N. Pérez,a Mátyás Mayer,c Cameron C. da Silva,a Felipe T. Martinsa and Pál Perjési*,b aInstituto de Química, Universidade Federal de Goiás, 74690-900 Goiânia-GO, Brazil bInstitute of Pharmaceutical Chemistry and cDepartment of Forensic Medicine, University of Pécs, H-7624 Pécs, Hungary 4’-Hydroxychalcones have been reported to possess several beneficial biological effects. Several lines of evidence accumulated to demonstrate increased biological activities of the Mannich base derivatives of the parent 4’-hydroxychalcones. Bioactivities of chalcones and related α,β-unsaturated ketones are frequently associated with their reactivity with cellular thiols, such as GSH. For comparison of GSH reactivity, two bis Mannich bases of two 4’-hydroxychalcones were synthesized and reacted with GSH under non-cellular conditions. Reversed-phase thin layer chromatography (RP-TLC) and reversed-phase high performance liquid chromatography (RP-HPLC) analysis showed formation of two polar products which structures were confirmed by RP-HPLC-ESI-MS (RP-HPLC-electrospray ionization mass spectrometry) as 1:1 chalcone-GSH adducts in each case. At pH values below 8.0, the two bis Mannich bases showed higher GSH reactivity than two 4’-hydroxychalcones. Influence of the nature of the amino groups, the ring-B substituents and pH of the medium on reactivity was also investigated. -

Copyrighted Material
Index Abbreviations receptor sites 202, 211 weak 122 amino acids, Table 500–1 muscarinic 413 weak, pH calculation 147–8 nucleic acids 551 nicotinic 413 Acid–base nucleotides, Table 551 as quaternary ammonium salt 202 catalysis, enzymes 516 peptides and proteins 504 Acetylcholinesterase equilibria 121 phosphates and diphosphates 277 enzyme mechanism 519–21 interactions, predicting 155–7 structural, Table 14 hydrolysis of acetylcholine 279 Acidic reagents, Table 157 ACE (angiotensin-converting enzyme) inhibitors 279 Acidity enzyme action 532 Acetyl-CoA carboxylase, in fatty acid acidity constant 122 inhibitors 532 biosynthesis 595 bond energy effects 125 Acetal Acetyl-CoA (acetyl coenzyme A) definition 121 in etoposide 233 as acylating agent 262 electronegativity effects 125 formation 229 carboxylation to malonyl-CoA 595, hybridization effects 128 polysaccharides as polyacetals 232 609 inductive effects 125–7 as protecting group 230 Claisen reaction 381 influence of electronic and structural Acetal and ketal enolate anions 373 features 125–34 cyclic, as protecting groups 481 in Krebs cycle 585 and leaving groups, Table 189 groups in sucrose 231 from β-oxidation of fatty acids 388 pKa values 122–5 Acetaldehyde, basicity 139 as thioester 262, 373 resonance / delocalization effects 129–34 Acetamide, basicity 139 Acetylene, bonding molecular orbitals 31 Acidity (compounds) Acetaminophen, see paracetamol Acetylenes, acidity 128 acetone 130 Acetoacetyl-CoA, biosynthesis from N-Acetylgalactosamine, in blood group acetonitrile 365 acetyl-CoA 392 -

Generation of a Structurally Diverse Library Through Alkylation and Ring
70 Acta Chim. Slov. 2013, 60, 70–80 Scientific paper Generation of a Structurally Diverse Library through Alkylation and Ring Closure Reactions Using 3-Dimethylamino-1-(thiophen-2-yl)propan-1-one Hydrochloride* Gheorghe Roman Petru Poni Institute of Macromolecular Chemistry, 41A Aleea Gr. Ghica Vodâ, Ias¸i 700487, Romania Corresponding author: E-mail: [email protected] Received: 01-06-2012 Abstract 3-Dimethylamino-1-(thiophen-2-yl)propan-1-one hydrochloride (2), a ketonic Mannich base derived from 2-acetyl- thiophene, was used as a starting material in different types of alkylation and ring closure reactions with a view to gene- rate a structurally diverse library of compounds. Compound 2 reacts with S-alkylated dithiocarbamic acid salts and aryl mercaptans to produce dithiocarbamates and thioethers, respectively. The dimethylamino moiety in compound 2 was exchanged with various aliphatic secondary and aromatic primary and secondary amines, whereas monocyclic NH-azoles such as pyrazole, imidazole, 1,2,4-triazole, and tetrazole were N-alkylated by compound 2. Ketones, pyrrole and in- doles have been the substrates subjected to C-alkylation reactions by compound 2. Ring closure reactions of compound 2 with a suitable bifunctional nucleophile yielded pyrazolines, pyridines, 2,3-dihydro-1,5-1H-benzodiazepines, 2,3- dihydro-1,5-1H-benzothiazepine, pyrimido[1,2-a]benzimidazole and 4-hydroxypiperidine derivatives. Keywords: Ketonic Mannich base, alkylation, amine exchange, cyclization. 1. Introduction through the replacement of the easily leaving dimethyla- mino group by various nucleophiles. Also, several cycliza- The chemistry of Mannich bases has drawn a great tions of the aforementioned Mannich base with bifunctio- deal of attention owing to the high synthetic potential and nal nucleophiles to 5-, 6- and 7-membered nitrogen-con- the outstanding applications of this class of compounds.2–4 taining heterocycles have been investigated. -

Comparative Total Syntheses of Strychnine
Comparative Total Syntheses of Strychnine N C D MacMillan Group Meeting N H O H A H B E Nathan Jui H N N H H F G July 22, 2009 O O O References: Pre-Volhardt: Bonjoch Chem. Rev. 2000, 3455. Woodward Tetrahedron, 1963, 247. Volhardt J. Am. Chem. Soc. 2001, 9324. Magnus J. Am. Chem. Soc. 1993, 8116. Martin J. Am. Chem. Soc. 2001, 8003. Overman J. Am. Chem. Soc. 1995, 5776. Bodwell Angew. Chem. Int. Ed. 2002, 3261. Kuehne J. Org. Chem. 1993, 7490. Mori J. Am. Chem. Soc. 2003, 9801. Kuehne J. Org. Chem. 1998, 9427. Shibasaki Tetrahedron 2004, 9569. Rawal J. Org. Chem. 1994, 2685. Fukuyama J. Am. Chem. Soc. 2004, 10246. Bosch, Bonjoch Chem. Eur. J. 2000, 655. Padwa Org. Lett. 2007, 279. History and Structure of (!)-Strychnine ! Isolated in pure form in 1818 (Pelletier and Caventou) ! Structural Determination in 1947 (Robinson and Leuchs) ! 24 skeletal atoms (C21H22N2O2) ! Isolated in pure form in 1818 (Pelletier and Caventou) N ! Over !25 07 -pruinbglisc,a 6ti-osntesr epoecrteaninteinrgs to structure ! Structural Determination in 1947 (Robinson and Leuchs) ! Notor!io u Ss ptoirxoicne (nletethr a(lC d-o7s) e ~10-50 mg / adult) N H H ! Over 250 publications pertaining to structure O O ! Comm!o nClyD uEs eridn gr osdyesntet mpoison ! Notorious poison (lethal dose ~10-50 mg / adult) ! Hydroxyethylidine Strychnos nux vomica ! $20.20 / 10 g (Aldrich), ~1.5 wt% (seeds), ~1% (blossoms) "For it's molecular size it is the most complex substance known." -Robert Robinson History and Structure of (!)-Strychnine ! 24 skeletal atoms (C21H22N2O2) N ! 7-rings, 6-stereocenters ! Spirocenter (C-7) N H H O O ! CDE ring system ! Hydroxyethylidine "For it's molecular size it is the most complex substance known." -Robert Robinson "If we can't make strychnine, we'll take strychnine" -R. -
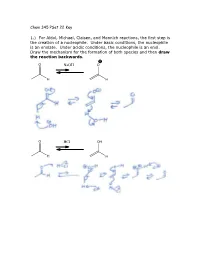
Chem 345 Pset 22 Key 1.) for Aldol, Michael, Claisen, and Mannich
Chem 345 PSet 22 Key 1.) For Aldol, Michael, Claisen, and Mannich reactions, the first step is the creation of a nucleophile. Under basic conditions, the nucleophile is an enolate. Under acidic conditions, the nucleophile is an enol. Draw the mechanism for the formation of both species and then draw the reaction backwards. O NaOH O H H O HCl OH H H 2.) In the case of a Mannich reaction, the electrophile (an imine) must also be made. Draw the mechanism for the forward and backward reaction: O MeNH2 N H H cat. AcOH H H 3.) When a secondary amine is used and the carbonyl has an enolizable proton, an enamine can be formed. Are enamines nucleophilic or electrophilic? Nucleophilic H O N N H2O H cat. AcOH H enamine N N H H enamine 4.) The following reaction is the Lobry-de-Bruyn-von-Ekenstein reaction. Don’t worry about the name. I only recently found out that that was the official name for the rearrangement leading from glucose to fructose or mannose and back again. I was taught it under a different name: the Carbohydrate Game. Under acidic or basic conditions, it is possible that the following sugars (glucose, fructose, and mannose) are in equilibrium with each other. Draw the acid catalyzed version and the base catalyzed version. (Hint: this is just a repeat of question 1.) O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose The sugars are drawn in a Fisher projection (or what I think of is a dead cat projection). -

Mannich Reaction
1. Introduction 1.1- Mannich Reaction The Mannich reaction is three component condensation in which a compound containing an active hydrogen atom is allowed to react with formaldehyde and an NH-amine derivative . Secondary amines rather than primary amines and ammonia are employed , the resulting product (Mannich Base ) is an amine compound having the N atom linked to the R substrate through a methylene group 1,2. The aminoalkylation of CH-acidic compounds was described by several authors as early as the 19th. century. However, it was Carl Mannich who was the first to recognize the enormous significance of this reaction , and it was he who extended the chemistry into a broad based synthetic methodology through systematic research. Since then this reaction that now carries his name has developed into one of the most important C-C bond-forming reactions in organic chemistry3,4. The Mannich reaction is a classical method for the preparation of β -aminoketones and aldehydes (Mannich bases) and, as such, is one of the most important basic reaction types in organic chemistry. It is the key step in the synthesis of numerous pharmaceuticals and natural products3,4. 1 The Mannich reaction can be presented by the following equation: The essential feature of the reaction is the replacement of the active hydrogen atom by an aminomethyl or substituted aminomethyl group. The symbolizes the active hydrogen component which includes ketones, aldehydes, acids, esters, phenols, acetylenes, picolines, nitroalkanes and quinolines1,2. 1.2- Mechanism of the Mannich reaction The mechanism of the Mannich reaction has been well investigated, the condensation reaction occurs in two steps: first, the amine reacts with formaldehyde to give condensation product (5 ), (6), ( 7) (step I) which then attacks the substrate R-H (step II) .The reaction does not normally follow the other possible route (step III and IV) ; however, some successful reaction between hydroxymethyl derivatives (8) and alkylamines to give Mannich bases (9) should be mentioned . -

Rearrangement Reactions
Rearrangement Reactions A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. 1, 2-Rearrangements A 1, 2-rearrangement is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1, 2 shift the movement involves two adjacent atoms but moves over larger distances are possible. In general straight-chain alkanes, are converted to branched isomers by heating in the presence of a catalyst. Examples include isomerisation of n-butane to isobutane and pentane to isopentane. Highly branched alkanes have favorable combustion characteristics for internal combustion engines. Further examples are the Wagner-Meerwein rearrangement: and the Beckmann rearrangement, which is relevant to the production of certain nylons: Pericyclic reactions A pericyclic reaction is a type of reaction with multiple carbon-carbon bonds making and breaking wherein the transition state of the molecule has a cyclic geometry and the reaction progresses in a concerted fashion. Examples are hydride shifts [email protected] and the Claisen rearrangement: Olefin metathesis Olefin metathesis is a formal exchange of the alkylidene fragments in two alkenes. It is a catalytic reaction with carbene, or more accurately, transition metal carbene complexintermediates. In this example (ethenolysis, a pair of vinyl compounds form a new symmetrical alkene with expulsion of ethylene. Pinacol rearrangement The pinacol–pinacolone rearrangement is a method for converting a 1,2-diol to a carbonyl compound in organic chemistry. The 1,2-rearrangement takes place under acidic conditions. -

Synthesis and Characterization of Some Novel Mannich Base Compounds
Synthesis and Characterization of Some Novel Mannich Base Compounds Dr. P. Sounthari Dr. P. R. Sivakumar Dr. P. Sounthari Dr. P.R. Sivakumar Assistant Professor, PG & Research Department of Department of Chemistry, Chemistry, PSG College of Arts and Government Arts Science, Coimbatore- College (Autonomous), 641 014. Coimbatore - 641 018, Tamil Nadu, India. ISBN 978-93-89631-02-9 ISBN 978-93-89631-04-3 (eBook) https://doi.org/10.34256/ioriip196 © IOR INTERNATIONAL PRESS. This book is an open access publication. Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. -

The Claisen Condensation
Lecture 19 The Claisen Condensation •• •• • • O • O • – • CH3COCH2CH3 • CH2 COCH2CH3 March 29, 2016 Chemistry 328N Exam Tomorrow Evening!! Review tonight 5PM -6PM Welch 1.316 Chemistry 328N Some “loose ends” before we go on Spectrosopy of acid derivatives A selective reduction for your tool box Chemistry 328N Reduction of Acid Derivatives Acids (page 679-681) Esters (page 738-739) Please work through the example on 738 Amides (page 739-742) Nitriles (page 742) Selective reductions with NaBH4 Chemistry 328N DIBAlH Diisobutylaluminum hydride (DIBAlH) at -78°C selectively reduces esters to aldehydes •at -78°C, the tetrahedral intermediate does not collapse and it is not until hydrolysis in aqueous acid that the carbonyl group of the aldehyde is liberated Stable at low temperature Chemistry 328N Infrared Spectroscopy C=O stretching frequency depends on whether the compound is an acyl chloride, anhydride, ester, or amide. C=O stretching frequency n O O O O O CH3CCl CH3COCCH3 CH3COCH3 CH3CNH2 1822 cm-1 1748 1736 cm-1 1694 cm-1 and 1815 cm-1 Chemistry 328N Infrared Spectroscopy Anhydrides have two peaks due to C=O stretching. One from symmetrical stretching of the C=O and the other from an antisymmetrical stretch. C=O stretching frequency n O O CH3COCCH3 1748 and 1815 cm-1 Chemistry 328N Infrared Spectroscopy Nitriles are readily identified by absorption due to carbon-nitrogen triple bond stretching that is “all alone” in the 2210-2260 cm-1 region. Chemistry 328N Hydrolysis and Decarboxylation Chemistry 328N t-Butyl esters Chemistry 328N t-Butyl esters Chemistry 328N t-Butyl ester hydrolysis Note which bond is broken in this hydrolysis !! Chemistry 328N Recall our discussion of the acidity of protons a to carbonyls The anion is stabilized by resonance The better the stabilization, the more acidic the a proton Acidity of a protons on“normal” aldehydes and ketones is about that of alcohols and less than water…pKa ~ 18-20 Some are far more acidic, i.e. -

The Claisen Condensation Is a Carbon–Carbon Bond Forming Reaction That Occurs Between Two Esters Or One Ester and Another Carb
Claisen Ester Condensation The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. Requirements ❖ At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). ❖ There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. ❖ The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. ❖ For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated. ❖ In mixed Claisen condensations, a non-nucleophilic base such as lithium diisopropylamide, or LDA, may be used, since only one compound is enolizable. ❖ LDA is not commonly used in the classic Claisen or Dieckmann condensations due to enolization of the electrophilic ester. ❖ The alkoxy portion of the ester must be a relatively good leaving group. ❖ Methyl and ethyl esters, which yields methoxide and ethoxide, respectively, are commonly used. Types Mechanism ❖ In the first step of the mechanism, an α-proton is removed by a strong base, resulting in the formation of an enolate anion, which is made relatively stable by the delocalization of electrons. ❖ Next, the carbonyl carbon of the (other) ester is nucleophilically attacked by the enolate anion. ❖ The alkoxy group is then eliminated (resulting in (re)generation of the alkoxide), and the alkoxide removes the newly formed doubly α-proton to form a new, highly resonance-stabilized enolate anion.