Band Width Selection Data from Near Infra-Red Spectral (NIRS
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -
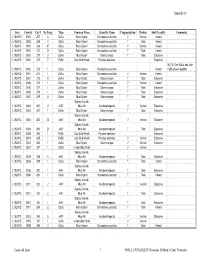
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2013): 4.438 New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India Souji.S1, Tresa Radhakrishnan2 Department of Aquatic Biology and Fisheries, University of Kerala, Thiruvananthapuram-695 581, Kariyavattom, Kerala, India Abstract: Arcacea family is economically important group of animals. Most of the species in this family are misplaced into invalid subgenera and Indian arcids are wanted a revision in systematic positon. In the case of Arcidae family; all of the species are treated under Anadara as main genera, however, some authors considered that the Tegillarca genus is only a sub genus of Arcidae family. Anadara is the commercially important genus of bivalves of Arcidae family. These two genera are confused by many taxonomists and some considered that the morphometric changes of Tegillarca are only the habitual adaptation. But the collected samples from the same habitat from the southern part of India is clearly demarked the distinction between Anadara species and Tegillarca species. In this paper the differences between these two genera are illustrated with the help of specimens from the same habitat and with the help of taxonomic literature of these genera. Species level classification was done based on the morphometric characters like peculiarities of (i) periostracum, (ii) cardinal area, (iii) umbo, (iv) adductor muscle scar and (v) pallial line. The specimens were collected from Neendakara, Vizhinjam and Kovalam along with the south west coast and Thiruchendur in Tamil Nadu, south east coast of India. -

CONTROL of PERKINSUS DISEASE in ABALONE R.J.G. Lester and C.J
CONTROL OF PERKINSUS DISEASE IN ABALONE R.J.G. Lester and C.J. Hayward Microbiology and Parasitology The University of Queensland Brisbane Queensland 4072 Project no. 2000/151 Final Report Control of Perkinsus disease in abalone R.J.G. Lester and C.J. Hayward Microbiology and Parasitology, The University of Queensland Brisbane, Queensland 4072 Final Report for FRDC Project no. 2000/151 Copyright Fisheries Research and Development Corporation and The University of Queensland, 2005 This work is copyright. Except as permitted under the Copyright Act 1968 (Cth), no part of this publication may be reproduced by any process, electronic or otherwise, without the specific written permission of the copyright owners. Neither may information be stored electronically in any form whatsoever without such permission. The Fisheries Research and Development Corporation plans, invests in and manages fisheries research and development throughout Australia. It is a statutory authority within the portfolio of the federal Minister for Agriculture, Fisheries and Forestry, jointly funded by the Australian Government and the fishing industry. ISBN 0-646-44752-1 Title: Control of Perkinsus Disease in Abalone Authors: Lester, Robert John Graham; Hayward, Craig James Date of Publication: 15 August, 2005 Publisher: University of Queensland - Marine Parasitology, Brisbane. Copies without Appendices 3 and 6 may be purchased from FRDC, PO Box 222, Deakin West, ACT 2600, Australia. Appendices 3 and 6 are published elsewhere as indicated. 2 Table of contents Non-technical -
FNC 37 Minutes Draft
FISH NAMES COMMITTEE The FRDC Standards Development Committee responsible for AS 5300 FNC 37 Minutes Meeting Details Date Wednesday 22 April 2020 Time AEST 10.00am – 12.30pm Venue Teleconference conducted by Zoom Contact Details CONTACTS Alan Snow [email protected] 0418 199 516 Project Manager Meaghan Dodd [email protected] 0438 423 597 Project Co-Investigator Gus Dannoun [email protected] 0419 528 733 Chair FNC Alan Snow Locked Bag 222 DIRECT 61 7 3390 6220 EMAIL [email protected] FNC Project Manager DEAKIN WEST ACT 2600 MOBILE 0418 199 516 WEB www.fishnames.com.au FRDC IS ACCREDITED TO DEVELOP AUSTRALIAN STANDARDS FOR THE FISHING AND AQUACULTURE INDUSTRY Meeting Minutes 1. OPENING OF MEETING ............................................................................................................................ 4 1.1 ATTENDANCE AND APOLOGIES .............................................................................................................. 4 1.2 NOTIFICATION OF PROXY VOTES ............................................................................................................ 5 1.3 NOTIFICATION OF OBSERVERS ............................................................................................................... 5 1.4 FNC MEMBERS CODE OF CONDUCT ...................................................................................................... 5 2. FISH NAMES COMMITTEE ...................................................................................................................... -

CRRC Bivalve and Littleneck Clam Culture Studies
5.0. Development of hatchery, nursery and growout methods for Nuttall’s cockle (Clinocardium nuttallii). During the 1995 shellfish surveys at the Alaskan Native villages of Tatitlek, Port Graham and Nanwalek, villagers repeatedly expressed a preference for cockles (Clinocardium nuttallii). Residents of Port Graham reported that cockles were common in the 1970’s and early 1980’s, but virtually disappeared several years before the Exxon Valdez oil spill. Very few cockles were observed in any of the quantitative or qualitative surveys conducted at Port Graham, Tatitlek, or Nanwalek. Excellent cockle habitat was observed in qualitative shellfish surveys at Port Graham and Tatitlek. The common cockle from the Eastern Atlantic (Cerastoderma edule) is prized in some areas of Europe and blood cockles of the genus Anadara are grown and marketed in Asia. However, Nuttall’s cockle, common in sandy intertidal areas of the eastern Pacific, is not cultivated and is not commonly harvested commercially. In part, that is because this bivalve does not keep well under refrigeration (author’s personal experience) and therefore has a limited commercial shelf-life. The result is that little work has been accomplished with respect to developing hatchery techniques for propagating this animal. A search of the ASFA and BIOSYS bibliographic databases revealed few citations dealing with the genus Clinocardium. All of those identified in the search were obtained from the University of Washington library system together with many of the references pertaining to other cockle species. 5.1. Background. In addition to being a favored food of Alaskan Natives, cockles appear to grow rapidly in Washington State. -

Invasive Alga Compromises Reproductive Development And
Sublethal effects on reproduction in native fauna: are females more vulnerable to biological invasion? Paul E. Gribben and Jeffrey T. Wright1 Corresponding Author Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, Sydney 2052, Australia. E-mail: [email protected] Ph: +61 2 9385 3690, Fax: +61 2 9385 2554 1Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, Sydney 2522 Australia Abstract: Although invasive species are a major threat to survivorship of native species, we know little about their sublethal effects. In soft-sediment marine systems, mat-forming invasive species often have positive effects, facilitating recruitment and enhancing the diversity and abundance of native invertebrates. However, because mat-forming invasive species change the habitat in which they invade, and benthic invertebrates are sensitive to environmental disturbance, important sublethal effects on native species may exist. Using a 1 model marine system we show that the widespread mat-forming invasive alga Caulerpa taxifolia (Vahl) C. Agardh has strong negative effects on the reproductive traits of a native bivalve Anadara trapezia (Deshayes, 1840) (e.g. timing of reproductive development and spawning, and follicle and gamete production) even though the invader has positive effects on recruitment. Moreover, gender specific responses occurred and indicated that females were more susceptible to invasion than males. Our results indicate that sublethal effects of an invasive species on reproductive traits will have severe consequences for fitness of the native species. Keywords: Caulerpa taxifolia, invasion biology, life history, reproductive traits, sex ratios. Introduction Invasive species have a range of direct and/or indirect lethal effects on native species. -

Bivalvia, Veneridae) (Carpenter, 1864
First description of growth, development and rearing of the sandy clam Chione cortezi (Bivalvia, Veneridae) (Carpenter, 1864) TATIANA N. OLIVARES-BAÑUELOS*, DANIELA RODRÍGUEZ-GONZÁLEZ, JAVIER GARCÍA-PAMARES & MARCO A. GONZÁLEZ-GÓMEZ Instituto de Investigaciones Oceanológicas, Universidad Autónoma de Baja California. Carretera Ensenada- Tijuana No. 3917, Fraccionamiento Playitas. C.P. 2286, Ensenada, Baja California, México * Corresponding author: [email protected] Abstract. Bivalve molluscs support important fisheries worldwide. In Baja California (BC), Mexico, a state with an extended coastline both in the Pacific and in the Gulf of California, fishing and aquaculture are important activities. Official records indicate that in 2015 the wild fishery contributed 1430 t of bivalves. Among endemic clams species exploited for human consumption in the Gulf of California, it´s found the sandy clam Chione cortezi (Carpenter, 1864). The growing demand has led to overfishing of the species, which makes it vulnerable and has put it at risk of disappearing from its natural habitat. The objectives of this study were to describe the external morphology, growth rate, and aspect ratio of larvae through juveniles of C. cortezi, under semi-commercial scale laboratory conditions. Culture yielded 5.70 million (M) of D-stage veliger larvae with a mean length (L) (± standard error) of 93.1 ± 0.5 µm and height (H) of 70.8 ± 0.6, 24 h post-fertilization (PF). Pediveligers (H = 235.4 ± 1.5 µm, L = 223.8 ±1.4) were observed after 9 days. Metamorphosis was epinephrine-induced on day 11, and postlarvae reached 2 mm by day 71 (H = 2391.0 ± 88.3 µm, L = 2164.0 ± 78.6). -

A Guide to Shells Commonly Found in Victorian Aboriginal Shell Middens
A GUIDE TO SHELLS COMMONLY FOUND IN VICTORIAN ABORIGINAL SHELL MIDDENS This guide has been developed as an aid to accurately completing the Shell Deposit component form of the Victorian Aboriginal Site Record cards. For the purposes of recording an Aboriginal midden for inclusion in the AAV Heritage Registry, shell material generally needs only to be identified to the Genus level and in some case (e.g Limpets and Chitons) to a Family or Order level, depending on the physical similarity of species. Only in cases where there is no ambiguity in the identification of individual species is recording to the species level expected. Shellfish can be divided into three classes; • Polyplacaphora - commonly called chitons, a group of animals covered with eight plates; • Gastropods - shellfish with a single, generally spiral shell, sometimes closed with a shelly "door" known as an operculum; and • Bivalves - shellfish having two shells e.g mussels.oysters, pipis;. The identification chart below provides the scientific grouping, any common names, a description of the shell(s) accompanied by a drawing of the shell or representative species and a description of the type of habitat where the animal can be found. Scientific names are subject to change and revision as research progresses. AAV used the CSIRO Fauna of Australia series for determining current scientific names. Further Reading: Beesley, P.L., G.J.B. Ross, & A. Wells (eds) 1998. Mollusca: the southern synthesis. Fauna of Australia Volume 5. CSIRO Publishing. Melbourne. Macpherson, J. H. & C. J . Gabriel. 1962. Marine Molluscs of Victoria. Melbourne University Press. Melbourne. Smith, B.J. -

03 Chapter 3 Vale
Chapter 3 Chapter 3: Previous Research in the Lower Macleay 3.1 Introduction This chapter provides a summary and review of the published research on the Lower Mac1eay. Although the research discussed here is not all of an archaeological nature, it is vital to the present research to understand how studies in the Macleay (by people from various disciplines) have built up the picture that we currently have of this area. The review is divided into three sections: first, the research published prior to 1970, which includes geological and geomorphological studies which were later taken up and used by archaeologists. This time period also includes the first extensive archaeological surveys carried out in the region. In the second section I examine the extensive archaeological excavations and analysis carried out by Connah and students from the University of New England in the 1970s, and also other surveys both archaeological and geological. While these reviews are primarily from published work, I also cite some unpublished documents such as B.A.(Hons) theses to obtain a full picture of previous research. The third section of the review focuses on work carried out since 1980. Following presentation and discussion of each time period of prior research, I provide a summary of the prevailing understanding of the time in regard to prehistoric Aboriginal occupation of the region and the environmental factors which may have affected that occupation. The final section of this chapter then deals with questionable assumptions and questions which remain unanswered despite all the research that has been carried out in the Lower Macleay. -

What's New in Bivalve Mollusc Pathology?
24, Bull. Eur. Ass. Fish Pathol., 36(1) 2016 What’s new in bivalve mollusc pathology Overview o articles published in the last two years A. Villalba Centro de Investigacións Mariñas (CIMA), Consellería do Mar da Xunta de ǰȱřŜŜŘŖȱȱȱǰȱDzȱ ¢ȱǰȱȱȱ Life Sciences, University of Alcalá, 28871 Alcalá de Henares, Spain Abstract This is an overview o articles on bivalve mollusc diseases published between the two last Inter- national Conerences on Diseases o Fish and Shellsh organised by the EAFP (September 2013 to September 2015). The need to limit the number o pages o this overview has involved selecting (and discarding) articles, prioritising by novelty and relevance, which entails an element o subjectivity. Journal special issues Highly relevant was the publication o special will be key reerences in their respective elds issues ocused on this eld by three journals or years. (in chronological order): (1) Microcell para- sites o molluscs (Diseases of Aquatic Organisms Immune response 110 (1-2), 2014), including recent research on Bivalve immune response captured much at- protistan parasites o molluscs in the genera tention in the reviewed period and numerous, Bonamia and Mikrocytos; (2) Molluscan Im- excellent articles were published, mostly on host munity (ȱǭȱęȱ ¢ 46 (1), transcriptomic/proteomic changes associated 2015), including reviews o many aspects o with pathogens/diseases (viruses, bacteria, pro- the immunology o molluscs as well as an tistan, stress, etc), showing the complexity o the announcement o the orthcoming eort to immune system o bivalve molluscs. Hopeully, sequence the genome o the eastern oyster, advances in this eld will be used to design Crassostrea virginica; and (3) Pathogens and strategies to ght diseases o economically rel- Disease Processes in Marine Molluscs (Journal evant molluscs, such as identiying molecular of Invertebrate Pathology 131, 2015), ocusing markers o disease resistance.