Developing Clam Aquaculture in Australia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Morphometry, Growth and Reproduction of an Atlantic Population of the Razor Clam Ensis Macha (Molina, 1782)*
SCI. MAR., 68 (2): 211-217 SCIENTIA MARINA 2004 Morphometry, growth and reproduction of an Atlantic population of the razor clam Ensis macha (Molina, 1782)* PEDRO J. BARÓN1,2, LUCIANO E. REAL2, NÉSTOR F. CIOCCO1,2 and MARÍA E. RÉ1 1 Centro Nacional Patagónico, CONICET, Boulevard Brown s/n, Puerto Madryn (9120), Chubut, Argentina. E-mail: [email protected] 2 Universidad Nacional de la Patagonia San Juan Bosco, Boulevard Brown 3700, Puerto Madryn (9120), Chubut, Argentina. SUMMARY: Ensis macha is a razor clam distributed throughout the coasts of southern Argentina and Chile. Even though it represents a valuable fishery resource, the exploitation of its Atlantic populations has begun only in recent years. This study provides the first estimates of growth rate, an interpretation of the reproductive cycle on the coast of the northern Argentine Patagonia and an analysis of the species morphometry. Growth was estimated by direct observation of growth rings on the valves by two observers. The reproductive cycle was interpreted by the analysis of temporal change of oocyte size frequency distributions. Parameter estimations for the von Bertalanffy equations respectively obtained by observers 1 -1 and 2 were 154 and 153.7 mm for L∞, 0.25 and 0.20 yr for k, and -0.08 and -0.72 yr for t0. Two spawning peaks were detect- ed: September-November 1999 and May-June 2000. However, mature females were found all year round. An abrupt change in the relationship between shell length and height was detected at 11.2 mm length. Key words: razor clam, morphometric relationships, growth, reproduction, Ensis macha, allometric growth. -

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Faunistic Assemblages of a Sublittoral Coarse Sand Habitat of the Northwestern Mediterranean
Scientia Marina 75(1) March 2011, 189-196, Barcelona (Spain) ISSN: 0214-8358 doi: 10.3989/scimar.2011.75n1189 Faunistic assemblages of a sublittoral coarse sand habitat of the northwestern Mediterranean EVA PUBILL 1, PERE ABELLÓ 1, MONTSERRAT RAMÓN 2,1 and MARC BAETA 3 1 Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta, 37-49, 08003 Barcelona, Spain. E-mail: [email protected] 2 Instituto Español de Oceanografía, Centre Oceanogràfic de les Balears, Moll de Ponent s/n, 07015 Palma de Mallorca, Spain. 3 Tecnoambiente S.L., carrer Indústria, 550-552, 08918 Badalona, Spain. SUMMARY: The sublittoral megabenthic assemblages of a northwestern Mediterranean coarse sandy beach exploited for the bivalve Callista chione were studied. The spatial and bathymetric variability of its distinctive faunal assemblages was characterised by quantitative sampling performed with a clam dredge. The taxa studied were Mollusca Bivalvia and Gastropoda, Crustacea Decapoda, Echinodermata and Pisces, which accounted for over 99% of the total biomass. Three well- differentiated species assemblages were identified: (1) assemblage MSS (Medium Sand Shallow) in medium sand (D50=0.37 mm) and shallow waters (mean depth =6.5 m), (2) assemblage CSS (Coarse Sand Shallow) in coarse sand (D50=0.62 mm) in shallow waters (mean depth =6.7 m), and (3) assemblage CSD (Coarse Sand Deep) in coarse sand (D50=0.64 mm) in deeper waters (mean depth =16.2 m). Assemblage MSS was characterised by the codominance of the bivalves Mactra stultorum and Acanthocardia tuberculata. C. chione was dominant in both density and biomass in assemblages CSS and CSD. -

Silicified Eocene Molluscs from the Lower Murchison District, Southern Carnarvon Basin, Western Australia
[<ecords o{ the Western A uslralian Museum 24: 217--246 (2008). Silicified Eocene molluscs from the Lower Murchison district, Southern Carnarvon Basin, Western Australia Thomas A. Darragh1 and George W. Kendrick2.3 I Department of Invertebrate Palaeontology, Museum Victoria, 1'.0. Box 666, Melbourne, Victoria 3001, Australia. Email: tdarragh(il.Illuseum.vic.gov.au :' Department of Earth and Planetary Sciences, Western Australian Museum, Locked Bag 49, Welshpool D.C., Western Australia 6986, Australia. 1 School of Earth and Ceographical Sciences, The University of Western Australia, 35 Stirling Highway, Crawlev, Western Australia 6009, Australia. Abstract - Silicified Middle to Late Eocene shallow water sandstones outcropping in the Lower Murchison District near Kalbarri township contain a silicified fossil fauna including foraminifera, sponges, bryozoans, solitary corals, brachiopods, echinoids and molluscs. The known molluscan fauna consists of 51 species, comprising 2 cephalopods, 14 bivalves, 1 scaphopod and 34 gastropods. Of these taxa three are newly described, Cerithium lvilya, Zeacolpus bartol1i, and Lyria lamellatoplicata. 25 of these molluscs are identical to or closely comparable with taxa from the southern Australian Eocene. The occurrence of this fauna extends the Southeast Australian Province during the Eocene from southwest Western Australia along the west coast north to at least 27° present day south latitude; consequently the province is here renamed the Southern Australian Province. Keywords: siliceous fossils, Eocene, Kalbarri, molluscs, new taxa, Carnarvon Basin, biogeography, Southern Australian Province. INTRODUCTION The source deposit, a pallid to ferruginous silicified Eocene marine molluscan assemblages from sandstone, forms a weakly defined, low breakaway coastal sedimentary basins in southern Australia trending N-S and sloping gently westward. -

You Are What You Eat: a Genomic Analysis of the Gut Microbiome of Captive and Wild Octopus Vulgaris Paralarvae and Their Zooplankton Prey
ORIGINAL RESEARCH published: 31 May 2017 doi: 10.3389/fphys.2017.00362 You Are What You Eat: A Genomic Analysis of the Gut Microbiome of Captive and Wild Octopus vulgaris Paralarvae and Their Zooplankton Prey Álvaro Roura 1, 2*, Stephen R. Doyle 1, 3, Manuel Nande 4, 5 and Jan M. Strugnell 1, 6 1 Department of Ecology, Environment and Evolution, La Trobe University, Melbourne, VIC, Australia, 2 Ecología y Biodiversidad Marina, Instituto de Investigaciones Marinas (CSIC), Vigo, Spain, 3 Parasite Genomic Group, Wellcome Trust Sanger Institute, Cambridge, United Kingdom, 4 Grupo de Acuicultura Marina, Instituto Español de Oceanografía, Vigo, Spain, 5 Departamento de Bioquímica, Genética e Inmunología, Universidad de Vigo, Vigo, Spain, 6 Marine Biology and Aquaculture, James Cook University, Townsville, QLD, Australia The common octopus (Octopus vulgaris) is an attractive species for aquaculture, however, several challenges inhibit sustainable commercial production. Little is known Edited by: about the early paralarval stages in the wild, including diet and intestinal microbiota, Giovanna Ponte, CephRes and SZN, Italy which likely play a significant role in development and vitality of this important life stage. Reviewed by: High throughput sequencing was used to characterize the gastrointestinal microbiome Muthugounder S. Shivakumar, of wild O. vulgaris paralarvae collected from two different upwelling regions off the coast Periyar University, India Andrea Tarallo, of North West Spain (n = 41) and Morocco (n = 35). These were compared to that Stazione Zoologica Anton Dohrn, Italy of paralarvae reared with Artemia for up to 25 days in captivity (n = 29). In addition, *Correspondence: the gastrointestinal microbiome of zooplankton prey (crabs, copepod and krill) was Álvaro Roura also analyzed to determine if the microbial communities present in wild paralarvae are [email protected] derived from their diet. -

Morphological Variations of the Shell of the Bivalve Lucina Pectinata
I S S N 2 3 47-6 8 9 3 Volume 10 Number2 Journal of Advances in Biology Morphological variations of the shell of the bivalve Lucina pectinata (Gmelin, 1791) Emma MODESTIN PhD of Biogeography, zoology and Ecology University of the French Antilles, UMR AREA DEV ABSTRACT In Martinique, the species Lucina pectinata (Gmelin, 1791) is called "mud clam, white clam or mangrove clam" by bivalve fishermen depending on the harvesting environment. Indeed, the individuals collected have differences as regards the shape and colour of the shell. The hypothesis is that the shape of the shell of L. pectinata (P. pectinatus) shows significant variations from one population to another. This paper intends to verify this hypothesis by means of a simple morphometric study. The comparison of the shape of the shell of individuals from different populations was done based on samples taken at four different sites. The standard measurements (length (L), width or thickness (E - épaisseur) and height (H)) were taken and the morphometric indices (L/H; L/E; E/H) were established. These indices of shape differ significantly among the various populations. This intraspecific polymorphism of the shape of the shell of P. pectinatus could be related to the nature of the sediment (granulometry, density, hardness) and/or the predation. The shells are significantly more elongated in a loose muddy sediment than in a hard muddy sediment or one rich in clay. They are significantly more convex in brackish environments and this is probably due to the presence of more specialised predators or of more muddy sediments. Keywords Lucina pectinata, bivalve, polymorphism of shape of shell, ecology, mangrove swamp, French Antilles. -

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -

National Review of Ostrea Angasi Aquaculture: Historical Culture, Current Methods and Future Priorities
National review of Ostrea angasi aquaculture: historical culture, current methods and future priorities Christine Crawford Institute of Marine and Antarctic Studies ! [email protected] " secure.utas.edu.au/profles/staff/imas/Christine-Crawford Executive summary Currently in Australia Ostrea angasi oysters (angasi) are being cultured on a small scale, with several farmers growing relatively small numbers of angasis on their predominately Sydney rock or Pacifc oyster farms. Very few farmers are culturing commercially viable quantities of angasi oysters. There are several reasons for this. Although angasi oysters occur in the intertidal zone, they are naturally most abundant in the subtidal and are less tolerant of fuctuating environmental conditions, especially temperature and salinity, than other oyster species. They also have a much shorter shelf life and start to gape after one to two days. Additionally, angasi oysters are susceptible to Bonamiosis, a parasitic disease which has caused major mortalities in several areas. Stress caused by extremes or a combination of factors such as high stocking densities, rough handling, poor food, high temperatures and low salinities have all been observed to increase the prevalence of Bonamiosis. Growth rates of angasi oysters have also been variable, ranging from two to four years to reach market size. From discussions with oyster famers, managers and researchers and from a review of the literature I suggest that the survival and growth of cultured angasi oysters could be signifcantly improved by altering some farm management practices. Firstly, growout techniques need to be specifcally developed for angasi oysters which maintain a low stress environment (not modifcations from other oysters). -

AEBR 114 Review of Factors Affecting the Abundance of Toheroa Paphies
Review of factors affecting the abundance of toheroa (Paphies ventricosa) New Zealand Aquatic Environment and Biodiversity Report No. 114 J.R. Williams, C. Sim-Smith, C. Paterson. ISSN 1179-6480 (online) ISBN 978-0-478-41468-4 (online) June 2013 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries TABLE OF CONTENTS EXECUTIVE SUMMARY ....................................................................................................... 1 1. INTRODUCTION ............................................................................................................ 2 2. METHODS ....................................................................................................................... 3 3. TIME SERIES OF ABUNDANCE .................................................................................. 3 3.1 Northland region beaches .......................................................................................... 3 3.2 Wellington region beaches ........................................................................................ 4 3.3 Southland region beaches ......................................................................................... -

Recent Trends in Marine Phycotoxins from Australian Coastal Waters
Review Recent Trends in Marine Phycotoxins from Australian Coastal Waters Penelope Ajani 1,*, D. Tim Harwood 2 and Shauna A. Murray 1 1 Climate Change Cluster (C3), University of Technology Sydney, Sydney, NSW 2007, Australia; [email protected] 2 Cawthron Institute, The Wood, Nelson 7010, New Zealand; [email protected] * Correspondence: [email protected]; Tel.: +61‐02‐9514‐7325 Academic Editor: Lucio G. Costa Received: 6 December 2016; Accepted: 29 January 2017; Published: 9 February 2017 Abstract: Phycotoxins, which are produced by harmful microalgae and bioaccumulate in the marine food web, are of growing concern for Australia. These harmful algae pose a threat to ecosystem and human health, as well as constraining the progress of aquaculture, one of the fastest growing food sectors in the world. With better monitoring, advanced analytical skills and an increase in microalgal expertise, many phycotoxins have been identified in Australian coastal waters in recent years. The most concerning of these toxins are ciguatoxin, paralytic shellfish toxins, okadaic acid and domoic acid, with palytoxin and karlotoxin increasing in significance. The potential for tetrodotoxin, maitotoxin and palytoxin to contaminate seafood is also of concern, warranting future investigation. The largest and most significant toxic bloom in Tasmania in 2012 resulted in an estimated total economic loss of ~AUD$23M, indicating that there is an imperative to improve toxin and organism detection methods, clarify the toxin profiles of species of phytoplankton and carry out both intra‐ and inter‐species toxicity comparisons. Future work also includes the application of rapid, real‐time molecular assays for the detection of harmful species and toxin genes. -

Physiological Effects and Biotransformation of Paralytic
PHYSIOLOGICAL EFFECTS AND BIOTRANSFORMATION OF PARALYTIC SHELLFISH TOXINS IN NEW ZEALAND MARINE BIVALVES ______________________________________________________________ A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in Environmental Sciences in the University of Canterbury by Andrea M. Contreras 2010 Abstract Although there are no authenticated records of human illness due to PSP in New Zealand, nationwide phytoplankton and shellfish toxicity monitoring programmes have revealed that the incidence of PSP contamination and the occurrence of the toxic Alexandrium species are more common than previously realised (Mackenzie et al., 2004). A full understanding of the mechanism of uptake, accumulation and toxin dynamics of bivalves feeding on toxic algae is fundamental for improving future regulations in the shellfish toxicity monitoring program across the country. This thesis examines the effects of toxic dinoflagellates and PSP toxins on the physiology and behaviour of bivalve molluscs. This focus arose because these aspects have not been widely studied before in New Zealand. The basic hypothesis tested was that bivalve molluscs differ in their ability to metabolise PSP toxins produced by Alexandrium tamarense and are able to transform toxins and may have special mechanisms to avoid toxin uptake. To test this hypothesis, different physiological/behavioural experiments and quantification of PSP toxins in bivalves tissues were carried out on mussels ( Perna canaliculus ), clams ( Paphies donacina and Dosinia anus ), scallops ( Pecten novaezelandiae ) and oysters ( Ostrea chilensis ) from the South Island of New Zealand. Measurements of clearance rate were used to test the sensitivity of the bivalves to PSP toxins. Other studies that involved intoxication and detoxification periods were carried out on three species of bivalves ( P. -
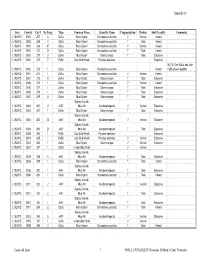
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud