Morphological Variations of the Shell of the Bivalve Lucina Pectinata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Lioconcha Castrensis Species Group (Bivalvia : Veneridae), with the Description of Two New Species
Molluscan Research 30(3): 117–124 ISSN 1323-5818 http://www.mapress.com/mr/ Magnolia Press The Lioconcha castrensis species group (Bivalvia : Veneridae), with the description of two new species SANCIA E.T. VAN DER MEIJ1, ROBERT G. MOOLENBEEK2 & HENK DEKKER2 1 Netherlands Centre for Biodiversity Naturalis (department of Marine Zoology), P.O. Box 9517, 2300 RA Leiden, The Nether- lands. Email: [email protected] (corresponding author) 2 Netherlands Centre for Biodiversity Naturalis (section Zoological Museum of Amsterdam), Mauritskade 57, 1092 AD Amsterdam, The Netherlands. Email: [email protected] Abstract Part of the genus Lioconcha Mörch, 1853 is reviewed. Species strongly resembling Lioconcha castrensis (Linnaeus, 1758) are discussed and two new species are described: Lioconcha arabaya n. sp. from the Northwest Indian Ocean and Lioconcha rumphii n. sp. from Thailand and Sumatra. These three species, together with Lioconcha macaulayi Lamprell & Healy, 2002, share many morphological similarities and we suspect them to be closely related. They are referred to as the Lioconcha cast- rensis species group. Furthermore, lectotypes of Venus castrensis Linnaeus, 1758, and Venus fulminea Röding, 1798, are desig- nated. The latter is considered a junior synonym of V. castrensis. Key words: Indo-Pacific, Mollusca, Persian Gulf, Red Sea, taxonomy Introduction between the anterior and posterior extremities, height is measured vertically from the umbo to the ventral margin and The delimitation within the tropical venerid genus Lioconcha total width (or inflation) is the greatest distance between the Mörch, 1853, is problematic, due to high levels of external surfaces of the paired valves. For an extensive list of intraspecific morphological variability and relatively few synonyms of figured specimens of Lioconcha castrensis we useful morphological characters (Lamprell and Healy 2002). -

Morphometry, Growth and Reproduction of an Atlantic Population of the Razor Clam Ensis Macha (Molina, 1782)*
SCI. MAR., 68 (2): 211-217 SCIENTIA MARINA 2004 Morphometry, growth and reproduction of an Atlantic population of the razor clam Ensis macha (Molina, 1782)* PEDRO J. BARÓN1,2, LUCIANO E. REAL2, NÉSTOR F. CIOCCO1,2 and MARÍA E. RÉ1 1 Centro Nacional Patagónico, CONICET, Boulevard Brown s/n, Puerto Madryn (9120), Chubut, Argentina. E-mail: [email protected] 2 Universidad Nacional de la Patagonia San Juan Bosco, Boulevard Brown 3700, Puerto Madryn (9120), Chubut, Argentina. SUMMARY: Ensis macha is a razor clam distributed throughout the coasts of southern Argentina and Chile. Even though it represents a valuable fishery resource, the exploitation of its Atlantic populations has begun only in recent years. This study provides the first estimates of growth rate, an interpretation of the reproductive cycle on the coast of the northern Argentine Patagonia and an analysis of the species morphometry. Growth was estimated by direct observation of growth rings on the valves by two observers. The reproductive cycle was interpreted by the analysis of temporal change of oocyte size frequency distributions. Parameter estimations for the von Bertalanffy equations respectively obtained by observers 1 -1 and 2 were 154 and 153.7 mm for L∞, 0.25 and 0.20 yr for k, and -0.08 and -0.72 yr for t0. Two spawning peaks were detect- ed: September-November 1999 and May-June 2000. However, mature females were found all year round. An abrupt change in the relationship between shell length and height was detected at 11.2 mm length. Key words: razor clam, morphometric relationships, growth, reproduction, Ensis macha, allometric growth. -

Shell Classification – Using Family Plates
Shell Classification USING FAMILY PLATES YEAR SEVEN STUDENTS Introduction In the following activity you and your class can use the same techniques as Queensland Museum The Queensland Museum Network has about scientists to classify organisms. 2.5 million biological specimens, and these items form the Biodiversity collections. Most specimens are from Activity: Identifying Queensland shells by family. Queensland’s terrestrial and marine provinces, but These 20 plates show common Queensland shells some are from adjacent Indo-Pacific regions. A smaller from 38 different families, and can be used for a range number of exotic species have also been acquired for of activities both in and outside the classroom. comparative purposes. The collection steadily grows Possible uses of this resource include: as our inventory of the region’s natural resources becomes more comprehensive. • students finding shells and identifying what family they belong to This collection helps scientists: • students determining what features shells in each • identify and name species family share • understand biodiversity in Australia and around • students comparing families to see how they differ. the world All shells shown on the following plates are from the • study evolution, connectivity and dispersal Queensland Museum Biodiversity Collection. throughout the Indo-Pacific • keep track of invasive and exotic species. Many of the scientists who work at the Museum specialise in taxonomy, the science of describing and naming species. In fact, Queensland Museum scientists -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

Estudio Cariológico En Moluscos Bivalvos
UNIVERSIDAD DE LA CORUÑA DEPARTAMENTO DE BIOLOGIA CELULAR Y MOLECULAR AREA DE GENETICA ESTUDIO CARIOLOGICO EN MOLUSCOS BIVALVOS MEMORIA que para optar al grado de Doctora presenta ANA MARIA INSUA POMBO La Coruña, Diciembre de 1992 N UNIVERSIDAD DE LA CORUNA DEPARTAMENTO DE BIOLOGIA CELULAR Y MOLECULAR AREA DE GENETICA ESTUDIO CARIOLOGICO EN MOLUSCOS BIVALVOS MEMORIA que para optar al grado de Doctora presenta ANA MARIA INSUA POMBO La Coruña, Diciembre de 1992 JOSEFINA MENDEZ FELPETO, PROFESORA TITULAR DE GENETICA DE LA UNIVERSIDAD DE LA CORUÑA, Y CATHERINE THIRIOT-QUIEVREUX, DIRECTORA DE INVESTIGACION EN EL CENTRE NATIONAL DE RECHERCHE SCIENTIFIQUE (FRANCIA), INFORMAN: Que el presente trabajo, ESTUDIO CARIOLOGICO EN MOLUSCOS BIVALVOS, que para optar al Grado de Doctora presenta De Ana María Insua Pombo, ha sido realizado bajo nuestra dirección y, considerándolo concluido, autorizamos su presentación al tribunal calificador. Villefranche-sur-Mer, a 7 de diciembre de 1992 La Coruña, a 9 de diciembre de 1992 c^_ Fdo. Catherine Thiriot-Quiévreux Fdo. Josefina Méndez Felpeto A Suso, Mano/a, G/oria e Leonor AGRADECIMIENTOS Este trabajo representa para mi un acúmulo de experiencias profesionales y personales que en gran medida transformaron mi vida de estos últimos años. Ahora ha Ilegado a su fin, pero esto no hubiese sido posible sin la ayuda, la colaboración y el aliento con el que he contado en el transcurso de su desarrollo. Por ello, deseo expresar mi más sincero agradecimiento a todas las personas que de una u otra manera contribuyeron a su realización. Josefina Méndez Felpeto se preocupó constantemente por mi formación investigadora desde el año 1986 en que me incorporó a su equipo y dirigió este trabajo con gran interés, respetando y apoyando en todo momento mis iniciativas. -

The Marine Mollusca of Suriname (Dutch Guiana) Holocene and Recent
THE MARINE MOLLUSCA OF SURINAME (DUTCH GUIANA) HOLOCENE AND RECENT Part II. BIVALVIA AND SCAPHOPODA by G. O. VAN REGTEREN ALTENA Rijksmuseum van Natuurlijke Historie, Leiden "The student must know something of syste- matic work. This is populary supposed to be a dry-as-dust branch of zoology. In fact, the systematist may be called the dustman of biol- ogy, for he performs a laborious and frequently thankless task for his fellows, and yet it is one which is essential for their well-being and progress". Maud D. Haviland in: Forest, steppe and tundra, 1926. CONTENTS Ι. Introduction, systematic survey and page references 3 2. Bivalvia and Scaphopoda 7 3. References 86 4. List of corrections of Part I 93 5. Plates 94 6. Addendum 100 1. INTRODUCTION, SYSTEMATIC SURVEY AND PAGE REFERENCES In the first part of this work, published in 1969, I gave a general intro- duction to the Suriname marine Mollusca ; in this second part the Bivalvia and Scaphopoda are treated. The system (and frequently also the nomen- clature) of the Bivalvia are those employed in the "Treatise on Invertebrate Paleontology, (N) Mollusca 6, Part I, Bivalvia, Volume 1 and 2". These volumes were issued in 1969 and contain the most modern system of the Bivalvia. For the Scaphopoda the system of Thiele (1935) is used. Since I published in 1968 a preliminary list of the marine Bivalvia of Suriname, several additions and changes have been made. I am indebted to Messrs. D. J. Green, R. H. Hill and P. G. E. F. Augustinus for having provided many new coastal records for several species. -

Trial Farming the Akoya Pearl Oyster, Pinctada Imbricata, in Portstephens
Trial farming the akoya pearl oyster, Pinctada imbricata, in Port Stephens, NSW Wayne A. O’Connor, Norman F. Lawler & Michael P. Heasman NSW Fisheries Port Stephens Fisheries Centre Private Bag 1, Nelson Bay, NSW, 2315, Australia Final Report to Australian Radiata Pty Ltd January 2003 NSW Fisheries Final Report Series No. 42 ISSN 1440-3544 Trial farming the akoya pearl oyster, Pinctada imbricata, in Port Stephens, NSW Wayne A. O’Connor, Norman F. Lawler & Michael P. Heasman NSW Fisheries Port Stephens Fisheries Centre Private Bag 1, Nelson Bay, NSW, 2315, Australia Final Report to Australian Radiata Pty Ltd January 2003 NSW Fisheries Final Report Series No. 42 ISSN 1440-3544 Table of Contents i TABLE OF CONTENTS TABLE OF CONTENTS................................................................................................................................. I ACKNOWLEDGMENTS ............................................................................................................................ III 1. NON-TECHNICAL SUMMARY ................................................................................................................ 5 1.1. Background .................................................................................................................................... 5 1.2. Need ............................................................................................................................................... 5 1.3. Objectives ..................................................................................................................................... -

Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora)
Gulf of Mexico Science Volume 34 Article 4 Number 1 Number 1/2 (Combined Issue) 2018 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution Martha Reguero Universidad Nacional Autónoma de México Andrea Raz-Guzmán Universidad Nacional Autónoma de México DOI: 10.18785/goms.3401.04 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Reguero, M. and A. Raz-Guzmán. 2018. Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution. Gulf of Mexico Science 34 (1). Retrieved from https://aquila.usm.edu/goms/vol34/iss1/4 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Reguero and Raz-Guzmán: Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Lagu Gulf of Mexico Science, 2018(1), pp. 32–55 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution MARTHA REGUERO AND ANDREA RAZ-GUZMA´ N Molluscs were collected in Laguna Madre from seagrass beds, macroalgae, and bare substrates with a Renfro beam net and an otter trawl. The species list includes 96 species and 48 families. Six species are dominant (Bittiolum varium, Costoanachis semiplicata, Brachidontes exustus, Crassostrea virginica, Chione cancellata, and Mulinia lateralis) and 25 are commercially important (e.g., Strombus alatus, Busycoarctum coarctatum, Triplofusus giganteus, Anadara transversa, Noetia ponderosa, Brachidontes exustus, Crassostrea virginica, Argopecten irradians, Argopecten gibbus, Chione cancellata, Mercenaria campechiensis, and Rangia flexuosa). -

Chemosymbiotic Bivalves from the Late Pliocene Stirone River Hydrocarbon Seep Complex in Northern Italy
Chemosymbiotic bivalves from the late Pliocene Stirone River hydrocarbon seep complex in northern Italy STEFFEN KIEL and MARCO TAVIANI Kiel, S. and Taviani, M. 2018. Chemosymbiotic bivalves from the late Pliocene Stirone River hydrocarbon seep complex in northern Italy. Acta Palaeontologica Polonica 63 (3): 557–568. Seven species of chemosymbiotic bivalves are described from the late Pliocene Stirone River hydrocarbon seep com- plex in northern Italy, including one new species and two in open nomenclature. The known species are the solemyid Acharax doderleini, the lucinids Lucinoma persolida and Megaxinus ellipticus, and the vesicomyid Isorropodon aff. perplexum; in open nomenclature we report two lucinids, including the largest species of Lucinoma known from the Italian Pliocene to date, and a strongly inflated, large Anodontia sp. The most abundant species at the Stirone seep com- plex is the lucinid Megaxinus stironensis sp. nov. This Pliocene seep fauna differs from that of the well-known Miocene “Calcari a Lucina” seep deposits by lacking large bathymodiolin mussels and vesicomyid clams; instead, the dominance of the lucinid Megaxinus stironensis gives this fauna a unique character. We speculate that at the Stirone seep complex, Megaxinus had occupied the ecological niche that Meganodontia occupied at the Miocene “Calcari a Lucina” seep sites in the Mediterranean basin, and that the dominance of Megaxinus could be a wide-spread feature of Pliocene chemosyn- thesis-based ecosystems in Mediterranean Pliocene. Key words: Bivalvia, Lucinidae, -

Siliqua Patula Class: Bivalvia; Heterodonta Order: Veneroida the Flat Razor Clam Family: Pharidae
Phylum: Mollusca Siliqua patula Class: Bivalvia; Heterodonta Order: Veneroida The flat razor clam Family: Pharidae Taxonomy: The familial designation of this (see Plate 397G, Coan and Valentich-Scott species has changed frequently over time. 2007). Previously in the Solenidae, current intertidal Body: (see Plate 29 Ricketts and Calvin guides include S. patula in the Pharidae (e.g., 1952; Fig 259 Kozloff 1993). Coan and Valentich-Scott 2007). The superfamily Solenacea includes infaunal soft Color: bottom dwelling bivalves and contains the two Interior: (see Fig 5, Pohlo 1963). families: Solenidae and Pharidae (= Exterior: Cultellidae, von Cosel 1993) (Remacha- Byssus: Trivino and Anadon 2006). In 1788, Dixon Gills: described S. patula from specimens collected Shell: The shell in S. patula is thin and with in Alaska (see Range) and Conrad described sharp (i.e., razor-like) edges and a thin profile the same species, under the name Solen (Fig. 4). Thin, long, fragile shell (Ricketts and nuttallii from specimens collected in the Calvin 1952), with gapes at both ends Columbia River in 1838 (Weymouth et al. (Haderlie and Abbott 1980). Shell smooth 1926). These names were later inside and out (Dixon 1789), elongate, rather synonymized, thus known synonyms for cylindrical and the length is about 2.5 times Siliqua patula include Solen nuttallii, the width. Solecurtus nuttallii. Occasionally, researchers Interior: Prominent internal vertical also indicate a subspecific epithet (e.g., rib extending from beak to margin (Haderlie Siliqua siliqua patula) or variations (e.g., and Abbott 1980). Siliqua patula var. nuttallii, based on rib Exterior: Both valves are similar and morphology, see Possible gape at both ends. -

Embryonic and Larval Development of Ensis Arcuatus (Jeffreys, 1865) (Bivalvia: Pharidae)
EMBRYONIC AND LARVAL DEVELOPMENT OF ENSIS ARCUATUS (JEFFREYS, 1865) (BIVALVIA: PHARIDAE) FIZ DA COSTA, SUSANA DARRIBA AND DOROTEA MARTI´NEZ-PATIN˜O Centro de Investigacio´ns Marin˜as, Consellerı´a de Pesca e Asuntos Marı´timos, Xunta de Galicia, Apdo. 94, 27700 Ribadeo, Lugo, Spain (Received 5 December 2006; accepted 19 November 2007) ABSTRACT The razor clam Ensis arcuatus (Jeffreys, 1865) is distributed from Norway to Spain and along the British coast, where it lives buried in sand in low intertidal and subtidal areas. This work is the first study to research the embryology and larval development of this species of razor clam, using light and scanning electron microscopy. A new method, consisting of changing water levels using tide simulations with brief Downloaded from https://academic.oup.com/mollus/article/74/2/103/1161011 by guest on 23 September 2021 dry periods, was developed to induce spawning in this species. The blastula was the first motile stage and in the gastrula stage the vitelline coat was lost. The shell field appeared in the late gastrula. The trocho- phore developed by about 19 h post-fertilization (hpf) (198C). At 30 hpf the D-shaped larva showed a developed digestive system consisting of a mouth, a foregut, a digestive gland followed by an intestine and an anus. Larvae spontaneously settled after 20 days at a length of 378 mm. INTRODUCTION following families: Mytilidae (Redfearn, Chanley & Chanley, 1986; Fuller & Lutz, 1989; Bellolio, Toledo & Dupre´, 1996; Ensis arcuatus (Jeffreys, 1865) is the most abundant species of Hanyu et al., 2001), Ostreidae (Le Pennec & Coatanea, 1985; Pharidae in Spain. -
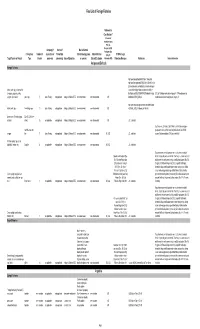
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S.