Effect of Some Myotropic Substances on Mollusc Hearts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -

Bivalvia: Veneridae) from Western Australia with Notes on Late Tertiary Species
Records of the Western Australian Museum 25: 369–377 (2010). A new species of the genus Proxichione (Bivalvia: Veneridae) from Western Australia with notes on Late Tertiary species Thomas A. Darragh Department of Invertebrate Palaeontology, Museum Victoria, G.P.O. Box 666, Melbourne Victoria 3001, Australia. E-mail: [email protected] Abstract Proxichione elimatula sp. nov. is described from the west and south coasts of Western Australia. Proxichione moondarae Darragh, 1965 is recorded from the Late Miocene of South Australia and Late Pliocene of Flinders Island, Tasmania. Proxichione cognata (Pritchard, 1903) is recorded from the Roe Calcarenite (Late Pliocene) of Western Australia. Keywords: new taxon, Murray Basin, Bass Basin. INTRODUCTION Australian Bight westwards and northwards to off Australian fossil species of Proxichione were Kalbarri, Western Australia) and the smallest of the revised and the genus differentiated from species P. persimilis (Iredale, 1930) (distributed from similar looking taxa of the Veneridae by Darragh central New South Wales northwards to southern (1965). Since that time specimens of a new living Queensland). This last species was designated as congeneric species have been accumulating in the the type species of Tigammona Iredale, 1930 (Figure collections of the Western Australian Museum. 1G-L). In addition, the geographical and stratigraphical range of the genus has been extended. Fossils of Proxichione belongs to a group of venerid bivalves Proxichione moondarae Darragh, 1965 have been with prominent comarginal and radial sculpture. collected in South Australia and on Flinders Island, The radial sculpture consists of simple, rounded Tasmania. Maxwell (1978) extended the range of ribs, whereas the comarginal sculpture consists of the genus to New Zealand by describing two taxa prominent erect lamellae, which are corrugated from the Duntroonian and Waitakian (Oligocene) where they cross the ribs and may be recurved of the South Island. -
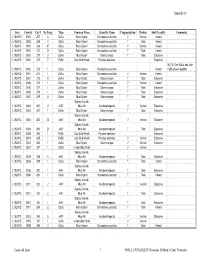
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2013): 4.438 New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India Souji.S1, Tresa Radhakrishnan2 Department of Aquatic Biology and Fisheries, University of Kerala, Thiruvananthapuram-695 581, Kariyavattom, Kerala, India Abstract: Arcacea family is economically important group of animals. Most of the species in this family are misplaced into invalid subgenera and Indian arcids are wanted a revision in systematic positon. In the case of Arcidae family; all of the species are treated under Anadara as main genera, however, some authors considered that the Tegillarca genus is only a sub genus of Arcidae family. Anadara is the commercially important genus of bivalves of Arcidae family. These two genera are confused by many taxonomists and some considered that the morphometric changes of Tegillarca are only the habitual adaptation. But the collected samples from the same habitat from the southern part of India is clearly demarked the distinction between Anadara species and Tegillarca species. In this paper the differences between these two genera are illustrated with the help of specimens from the same habitat and with the help of taxonomic literature of these genera. Species level classification was done based on the morphometric characters like peculiarities of (i) periostracum, (ii) cardinal area, (iii) umbo, (iv) adductor muscle scar and (v) pallial line. The specimens were collected from Neendakara, Vizhinjam and Kovalam along with the south west coast and Thiruchendur in Tamil Nadu, south east coast of India. -

The Marine Mollusca of Suriname (Dutch Guiana) Holocene and Recent
THE MARINE MOLLUSCA OF SURINAME (DUTCH GUIANA) HOLOCENE AND RECENT Part II. BIVALVIA AND SCAPHOPODA by G. O. VAN REGTEREN ALTENA Rijksmuseum van Natuurlijke Historie, Leiden "The student must know something of syste- matic work. This is populary supposed to be a dry-as-dust branch of zoology. In fact, the systematist may be called the dustman of biol- ogy, for he performs a laborious and frequently thankless task for his fellows, and yet it is one which is essential for their well-being and progress". Maud D. Haviland in: Forest, steppe and tundra, 1926. CONTENTS Ι. Introduction, systematic survey and page references 3 2. Bivalvia and Scaphopoda 7 3. References 86 4. List of corrections of Part I 93 5. Plates 94 6. Addendum 100 1. INTRODUCTION, SYSTEMATIC SURVEY AND PAGE REFERENCES In the first part of this work, published in 1969, I gave a general intro- duction to the Suriname marine Mollusca ; in this second part the Bivalvia and Scaphopoda are treated. The system (and frequently also the nomen- clature) of the Bivalvia are those employed in the "Treatise on Invertebrate Paleontology, (N) Mollusca 6, Part I, Bivalvia, Volume 1 and 2". These volumes were issued in 1969 and contain the most modern system of the Bivalvia. For the Scaphopoda the system of Thiele (1935) is used. Since I published in 1968 a preliminary list of the marine Bivalvia of Suriname, several additions and changes have been made. I am indebted to Messrs. D. J. Green, R. H. Hill and P. G. E. F. Augustinus for having provided many new coastal records for several species. -

56/2 · 2015 FOLIA BIOLOGICA ET GEOLOGICA Ex: Razprave Razreda Za Naravoslovne Vede Dissertationes Classis IV (Historia Naturalis)
FOLIA BIOLOGICA ET GEOLOGICA = Ex RAZPRAVE IV. RAZREDA SAZU issn 1855-7996 · Letnik / Volume 56 · Številka / Number 2 · 2015 ISSN 1855-7996 VSEBINA / CONTENTS RAZPRAVE / ESSAYS Vasja Mikuž, Aleš Šoster & Vili Rakovc 5 Paleogenske školjke iz Poljšice pri Podnartu 5 Paleogene bivalves from Poljšica near Podnart, Slovenia Vasja Mikuž, Aleš Šoster & Špela Ulaga 57 Druga najdba sipine kosti (Sepiidae) v miocenskih skladih kamnoloma plesko 57 The second find of cuttlefish shell (Sepiidae) in Miocene beds of the plesko quarry, Slovenia Aleš Šoster & Vasja Mikuž 69 Ostanek ustnače (Labridae) iz spodnjemiocenskih plasti Klanca nad Dobrno 69 The remain of wrasse (Labridae) from Early Miocene Klanc beds above Dobrna Vasja Mikuž, Aleš Šoster, France Stare & Milan Sukič Prekmurski 77 Megalodonovi zobje iz miocenskih laporovcev Virštanja 77 Megalodon teeth from Miocene marlstone at Virštanj, Slovenia Vasja Mikuž, Aleš Šoster & Mihael Ravnjak 109 Kostni ostanki delfina (Odontoceti) iz meljevcev gradbene jame hidroelektrarne Brežice 109 The dolphin bone remains (Odontoceti) from siltstone in excavation pit for the hydroelectric station Brežice, Slovenia FOLIA BIOLOGICA ET GEOLOGICA 56/2 – 2015 56/2 GEOLOGICA ET BIOLOGICA FOLIA Vasja Mikuž, Jernej Pavšič & Aleš Šoster 125 Skeletni ostanki sesalca v sarmatijskem laporovcu iz okolice Zidanega Mosta 125 The mammal skeleton remains in Sarmatian marlstone from vicinity of Zidani Most, Slovenia 56/2 · 2015 FOLIA BIOLOGICA ET GEOLOGICA Ex: Razprave razreda za naravoslovne vede Dissertationes classis IV (Historia -

ICAR-NAARM-Annual-Report 2016-17
funs'kd dh dye ls eq>s] Hkkd`vuqi & jk"Vªh; d`f"k vuqla/kku izca/k vdkneh ¼NAARM½ dh o"kZ 2016&17 dh okf"kZd fjiksVZ izLrqr djrs gq, izlUurk gks jgh gSA vdkneh } kjk jk"Vªh; d`f"k vuqla/kku ,oa f'k{kk iz.kkyh ¼NARES½ esa ekuo lalk/ku dh n{krk vkSj {kerkvksa dks c<+kus esa ,d egRoiw.kZ Hkwfedk fuHkkbZ tkrh gSA o"kZ 2016&17 ds nkSjku vdkneh }kjk dh xbZa igyksa dk Qksdl bu iz;klksa dks vkxs c<+kus ij dsfUnzr jgkA eSa] MkW- Mh- jkek jko ,oa MkW- vkj- dYiuk 'kkL=h dks c/kkbZ nsrk gwa ftUgksaus o"kZ 2016&17 ds nkSjku vdkneh ds funs'kd ds :i esa vius usr`Ro esa fofHkUu MkW- िसएच. Jhfuokl jko igyksa dks lkdkj :i fn;kA lkFk gh eSa vdkneh ds fotu vkSj fe'ku dks funs'kd vkxs c<+kus esa n'kkZ, x, mRlkg o meax ds fy, lHkh ladk; lnL;ksa] LVkQ ,oa Nk=ksa dh ljkguk djrk gwa A tSlk fd ge 'kh?kz gh 42osa o"kZ esa izos'k djus okys gSa] eSa ukeZ ifjokj ds lHkh lnL;ksa ds fy, lokZf/kd eaxye; o"kZ dh dkeuk djrk gwaA lkHkkj] fnukad % 10 tqykbZ] 2017 MkW- िसएच. Jhfuokl jko LFkku % gSnjkckn From the DIRECTOR’S DESK am happy to present the Annual Report of ICAR– NAARM for the year 2016-17. The Academy plays a pivotal role in enhancing the competencies and I capabilities of the human resources in the National Agricultural Research and Education System (NARES). -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

A Newspeciesofjapanesechama
VENUS 64 (l-2): 11-21, 2005 A New Species of Japanese Chama (Bivalvia: Heterodonta) with with a Calcitic Outermost Layer Naoto Hamada1 and Akihiko Matsukuma2 2 1Kumamoto University, 2-39-1, Kurokami, Kumamoto 860-8555, Japan めushu Universi ηMuseum, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan; matukuma@museum. わ1ushu-u.ac.j Abstract: Abstract: A new species of the Chamidae from Japan is described under the name of Chama cerinorhodon cerinorhodon n. sp. Chama cerinorhodon has hitherto been misidentified as Chama fragum Reeve, Reeve, 1846, from its superficial morphological characters. Howev 巴r, it is here reveal 巴d that this this species is sufficiently distinct from true C. fragum in shell mineralogy and shell structure to to warrant separate status. Chama cerinorhodon has a calcitic outermost shell layer in addition to to aragonitic inner and median shell lay 巴rs, whereas C. jトagum has only aragonitic inn 巴r and outer outer shell layers. Chama cerinorhodon is closely related to the eastern Pacific species Chama arcana arcana Bern 紅 d, 1976, and Chama pellucida Broderip, 1835, in shell morphology, mineralogy, and and structure. Keywords: Keywords: Chama, new species, calcitic lay 巴r Introduction Introduction The superfamily Chamoidea is represented by the single family Chamidae Lamarck, 1809, with with approximately 70 living species world-wide (Bernard, 1976). The taxonomy is confused at not not only species but also higher levels (Matsukuma, 1996). Ecologically, almost all chamids are sessile sessile in the epifaunal benthos and usually attach themselves to a hard substratum by one or the other other valve. Some exceptions exist, such as the secondary free living genus Arcinella Schumacher, 1817, 1817, of Central America, but they too undergo a brief phase of cementation at an early stage of of life. -

Zooarchaeology of Cinnamon Bay, St. John, Us Virgin Islands
Bull. Fla. Mus. Nat. Hist. (2003) 44(1): 131 -158 131 ZOOARCHAEOLOGY OF CINNAMON BAY, ST. JOHN, U.S. VIRGIN ISLANDS: PRE-COLUMBIAN OVEREXPLOITATION OF ANIMAL RESOURCES Irvy R. Quitmyeri The zooarchaeological remains from a stratigraphic sequence excavated from the ceremonial site of Cinnamon Bay, St. John, U.S. Virgin Islands, were studied. The samples were recovered using a fine-gauge (1/16 in) screen. During the course of this study, 443 minimum numbers of individuals and 99 species of vertebrates and invertebrates were identified. The fauna was analyzed by estimating the trophic level of reef, inshore, and pelagic zooarchaeological components from three strata representing the Monserrate (ca. A.D. 950), Santa Elena (ca. A.D. 570), and Chican (ca. A.D. 460) ceramic periods. The trophic level model shows an initial increase in the trophic level of taxa from the reef between the Monserrate and Santa Elena periods. This initial increase corresponds to the exponential growth of midden density. Relative to the earlier faunal assemblages, midden density and the mean trophic level of reef resources declines during the Chican period. Greater reliance on pelagic species from the deeper waters offshore and the increased use of mollusks from inshore habitats is also seen. The data show that at low levels of cultural complexity humans can alter their environments. This is particularly true of island biota where biological reservoirs are small. Key words: candy, Caribbean, island biogeography, trophic level, zooarchaeology This chapter presents a study of well-recovered Caribbean pre-Columbian people is not well zooarchaeological remains from the Cinnamon Bay site understood and should be considered in its formative (12Vam-2-3), St. -

Proceedings of the United States National Museum, III
* SYNOPSIS OF thp: family venerid.t^ and of the NORTH AMERICAN RECENT SPECIES. B}^ WiLiJAM Hkai;ky Dall, Honontrji ('iirator, Division of Mollnsks. This synopsis is one of a series of similar summaries of the families of bivalve mollusks which have been prepared by the writer in the course of a revision of our Peleeypod fauna in the light of th(^ material accumulated in the collections of the United States National Museum. While the lists of species are made as complete as possible, for the coasts of the United States, the list of those ascribed to the Antilles, Central and South America, is pro])ably subject to considerable addi- tions when the fauna of these regions is better known and the litera- ture more thoroughly sifted. No claim of completeness is therefore made for this portion of the work, except when so expressly stated. So many of the southern forms extend to the verge of our territory that it was thought well to include those known to exist in the vicinity when it could l)e done without too greatly increasing the labor involved in the known North American list. The publications of authors included in the bibliograph}' which follows are referred to by date in the text, but it may be said that the full explanation of changes made and decisions as to nomenclature arrived at is included in the memoir on the Tertiary fauna of Florida in course of pul)lication by the Wagner Institute, of Philadelphia, for the writer, forming the third volume of their transactions. The rules of nomenclature cited in Part 111 of that work (pp.