Marine Bivalve Molluscs E'
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -

Os Nomes Galegos Dos Moluscos
A Chave Os nomes galegos dos moluscos 2017 Citación recomendada / Recommended citation: A Chave (2017): Nomes galegos dos moluscos recomendados pola Chave. http://www.achave.gal/wp-content/uploads/achave_osnomesgalegosdos_moluscos.pdf 1 Notas introdutorias O que contén este documento Neste documento fornécense denominacións para as especies de moluscos galegos (e) ou europeos, e tamén para algunhas das especies exóticas máis coñecidas (xeralmente no ámbito divulgativo, por causa do seu interese científico ou económico, ou por seren moi comúns noutras áreas xeográficas). En total, achéganse nomes galegos para 534 especies de moluscos. A estrutura En primeiro lugar preséntase unha clasificación taxonómica que considera as clases, ordes, superfamilias e familias de moluscos. Aquí apúntase, de maneira xeral, os nomes dos moluscos que hai en cada familia. A seguir vén o corpo do documento, onde se indica, especie por especie, alén do nome científico, os nomes galegos e ingleses de cada molusco (nalgún caso, tamén, o nome xenérico para un grupo deles). Ao final inclúese unha listaxe de referencias bibliográficas que foron utilizadas para a elaboración do presente documento. Nalgunhas desas referencias recolléronse ou propuxéronse nomes galegos para os moluscos, quer xenéricos quer específicos. Outras referencias achegan nomes para os moluscos noutras linguas, que tamén foron tidos en conta. Alén diso, inclúense algunhas fontes básicas a respecto da metodoloxía e dos criterios terminolóxicos empregados. 2 Tratamento terminolóxico De modo moi resumido, traballouse nas seguintes liñas e cos seguintes criterios: En primeiro lugar, aprofundouse no acervo lingüístico galego. A respecto dos nomes dos moluscos, a lingua galega é riquísima e dispomos dunha chea de nomes, tanto específicos (que designan un único animal) como xenéricos (que designan varios animais parecidos). -
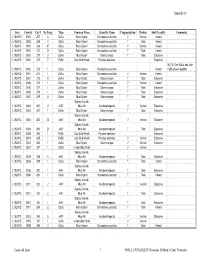
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2013): 4.438 New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India Souji.S1, Tresa Radhakrishnan2 Department of Aquatic Biology and Fisheries, University of Kerala, Thiruvananthapuram-695 581, Kariyavattom, Kerala, India Abstract: Arcacea family is economically important group of animals. Most of the species in this family are misplaced into invalid subgenera and Indian arcids are wanted a revision in systematic positon. In the case of Arcidae family; all of the species are treated under Anadara as main genera, however, some authors considered that the Tegillarca genus is only a sub genus of Arcidae family. Anadara is the commercially important genus of bivalves of Arcidae family. These two genera are confused by many taxonomists and some considered that the morphometric changes of Tegillarca are only the habitual adaptation. But the collected samples from the same habitat from the southern part of India is clearly demarked the distinction between Anadara species and Tegillarca species. In this paper the differences between these two genera are illustrated with the help of specimens from the same habitat and with the help of taxonomic literature of these genera. Species level classification was done based on the morphometric characters like peculiarities of (i) periostracum, (ii) cardinal area, (iii) umbo, (iv) adductor muscle scar and (v) pallial line. The specimens were collected from Neendakara, Vizhinjam and Kovalam along with the south west coast and Thiruchendur in Tamil Nadu, south east coast of India. -

THE NAUTILUS [Vol
2 2 THE NAUTILUS [Vol. 69 (1) separated from each other by five centimeters. The snail was expanded with its head oriented away from the clam. When placed on the sand, the clam showed no activity during the next 30 minutes after which observations were discontinued. All but the lower (anterior) end of the body of the clam was covered by an envelope of slime secreted by the foot of the snail. It seems clear that P. duplicatus capturesEnsis directus by approaching it below the surface of the substratum and by ir ritating the lower portion so that it retreats upward. The snail then coats the razor clam with an envelope of slime which ap pears to have anesthetic properties. Successful capture proba bly depends on the ability of the snail to maintain contact with its prey until anesthesia takes place. ON THE OCCURRENCE OF THE NUDIBRANCH ALDERIA MODESTA (LOVÉN, 1844) ON THE CENTRAL CALIFORNIAN COAST By CADET HAND and JOAN STEINBERG Department of Zoology, University of California, Berkeley Alderia modesta (Loven, 1844) has long been known from the coasts of northern Europe. It has been recorded from as far north as the Trondheim Fjord in Norway (Norman, 1893), south to Skibbereen in Ireland (Allman, 1845) and on the French coast (Gollien, 1929). Therefore, it has been of con siderable interest to us to find well-established populations of an Alderia in two localities on the central Californian coast. Through the kindness of Monsieur G. Van Put of the Royal Institute of Natural Sciences of Belgium in Brussels and Dr. -

New Alternative Fishing Gear Suggestions for Trap Fisheries from the Waste Recycle Materials
MARINE SCIENCE AND TECHNOLOGY BULLETIN VOLUME: 8 ISSUE: 2 DECEMBER 2019 Editor-in-Chief A.Y. Sönmez Kastamonu University, Turkey Co-Editors S. Kale Çanakkale Onsekiz Mart University, Turkey S. Bilen Kastamonu University, Turkey E. Terzi Kastamonu University, Turkey A.E. Kadak Kastamonu University, Turkey Editorial Board A.O. Sudrajat Institut Pertanian Bogor, Indonesia B. Bayraklı Sinop University, Turkey D. Güroy Yalova University, Turkey F. Şen Yüzüncü Yıl University, Turkey G. Biswas Kakdwip Research Centre of Central Institute, India H.H. Atar Ankara University, Turkey İ. Altınok Karadeniz Technical University, Turkey M. Elp Kastamonu University, Turkey M. Sazykina Southern Federal University, Russia M. Gökoğlu Akdeniz University, Turkey M.N. Khan University of the Punjab, Pakistan S. Beqiraj University of Tirana, Albania S. Acarlı Çanakkale Onsekiz Mart University, Turkey S.Z.B. Halun Mindanao State University, Philippines S. Uzunova Institute of Fishing Resources, Bulgaria S. Özdemir Sinop University, Turkey Ş. Kayış Recep Tayyip Erdoğan University, Turkey Ş. Yıldırım Ege University, Turkey T. Yanık Atatürk University, Turkey W. Leal Filho Hamburg University of Applied Sciences, Germany MARINE SCIENCE AND TECH NOLOGY BULLETIN Acknowledgement AUTHOR GUIDELINES Keep these to the absolute minimum and placed before the reference section. References Manuscripts must be submitted to the journal in electronic version only via Citation in text; online submission system at http://dergipark.org.tr/masteb. Please ensure that each reference cited in the text is also presented in the reference list. Cite literature in the text in chronological, followed by Types of Paper alphabetical order like these examples “(Mutlu et al., 2012; Biswas et al., 2016; Original research papers; review articles; short communications; letters to the Yanık and Aslan, 2018)”. -

Occurrence of the Hairy Ark Cockle (Anadara Gubernaculum, Reeve 1844) in Mayangan Coastal Waters, Subang – Province West Java: New Distribution Record of Indonesia
Asian Journal of Conservation Biology, December 2016. Vol. 5 No. 2, pp. 70-74 AJCB: FP0075 ISSN 2278-7666 ©TCRP 2016 Occurrence of the hairy ark cockle (Anadara gubernaculum, Reeve 1844) in Mayangan coastal waters, Subang – Province West Java: new distribution record of Indonesia Dewi Fitriawati1*, Nurlisa Alias Butet2 and Yusli Wardiatno2 1Master Program in Aquatic Resources Management, Graduate School of Bogor Agricultural University, Kampus IPB Dramaga – Bogor 16680, Indonesia 2Department of Aquatic Resources Management, Faculty of Fisheries and Marine Science, Bogor Agricultural University, Kampus IPB Dramaga – Bogor 16680, Indonesia (Accepted November 25, 2016) ABSTRACT Thirty six specimens of the hairy ark cockle (Anadara gubernaculum Reeve, 1844) were collected from Mayangan waters, Subang of Province West Java, during marine biodiversity survey along northern coast of Java Island, Indonesia. Previous records of the cockle showed its occurrence in Cilincing, northern coast of Jakarta, Semarang coastal waters of Province Central Java, and Sidoarjo coastal waters located in northern part of Province East Java. The identification of the species was performed by morphological characters and is supported by molecular analysis. This finding represents a new distribution record of the species in Indonesia. Keywords: Arcidae, bivalves, first record, mollusc, north Java. INTRODUCTION A.gubernaculum from Mayangan waters, Subang – Province West Java, Indonesia. At the same time it is a As an aquatic biodiversity hotspot, Indonesia has rich and diverse marine organisms, one of them is the cock- new distribution record of the species in Indonesian les group of Arcidae. The family Arcidae is a large and waters. diverse family of marine bivalve distributed worldwide in warm seas of tropical areas. -

Ponderous Ark Aquaculture in Florida
The Potential of Blood Ark and Ponderous Ark Aquaculture in Florida Results of Spawning, Larval Rearing, Nursery and Growout Trials Leslie N. Sturmer, Jose M. Nuñez, R. LeRoy Creswell, and Shirley M. Baker TP-169 SEPTEMBER 2009 Cover illustration: Ann Meyers This research was supported by the Cooperative State Research, Education, and Extension Service of the U.S. Department of Agriculture (USDA) under USDA Special Research Grant No. 2002-3445-11946; and by the National Sea Grant College Program of the U.S. Department of Commerce’s National Oceanic and Atmosphere Administration (NOAA) under NOAA Grant No. NA06 OAR-4170014. The views expressed are those of the authors and do not necessarily reflect the views of these organizations. Additional copies are available by contacting: Shellfish Aquaculture Extension Program Florida Sea Grant University of Florida University of Florida PO Box 89 PO Box 110409 Cedar Key, FL 32625-0089 Gainesville, FL 32622-0409 (352)543-5057 (352) 392-2801 www.flseagrant.org TP 169 September 2009 The Potential of Blood Ark (Anadara ovalis) and Ponderous Ark (Noetia ponderosa) Aquaculture in Florida Results of Spawning, Larval Rearing, Nursery, and Growout Trials Leslie N. Sturmer Shellfish Aquaculture Extension Program Cooperative Extension Service Institute of Food and Agricultural Sciences University of Florida Cedar Key Jose M. Nuñez The Whitney Laboratory for Marine Bioscience University of Florida St. Augustine R. LeRoy Creswell Florida Sea Grant College Program Institute of Food and Agricultural Sciences University of Florida Fort Pierce Shirley M. Baker Fisheries and Aquatic Sciences Program School of Forest Resources and Conservation Institute of Food and Agricultural Sciences University of Florida Gainesville September 2009 TP 169 ii Preface In November 1999, a workshop on New Molluscs for Aquaculture was conducted by the University of Florida Cooperative Extension Service, Florida Sea Grant, and the Florida Department of Agriculture and Consumer Services. -

Exploring Options for an Olympic Coast Ocean Acidification Sentinel Site (Oases)
Exploring Options for an Olympic Coast Ocean Acidification Sentinel Site (OASeS) Workshop Proceedings September 2016 Contents Background ..................................................................................................................................... 1 Olympic Coast ............................................................................................................................ 1 Ocean Acidification .................................................................................................................... 1 Ocean Acidification Sentinel Site (OASeS) Workshop Background ......................................... 2 Workshop Goals.......................................................................................................................... 3 Panel Discussion Summaries .......................................................................................................... 3 Science in National Marine Sanctuaries and OCNMS ............................................................... 3 i. i.Panelists: Steve Giddings, Liam Atrim, Scott Noakes, Lee Whitford Partners and Activities ................................................................................................................ 5 i. Panelists: Libby Jewett, Jan Newton, Richard Feely, Steve Fradkin, Paul McElhany, Joe Shumacker Education and Communication ................................................................................................... 7 i. Panelists: Laura Francis, Christopher Krembs, Jacqueline Laverdure, Angie -

Anadara Kagoshimensis (Mollusca: Bivalvia: Arcidae)
Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net http://dx.doi.org/10.12681/mms.2076 Anadara kagoshimensis (Mollusca: Bivalvia: Arcidae) in the Adriatic Sea: morphological analysis, molecular taxonomy, spatial distribution, and prediction PIERLUIGI STRAFELLA1, ALICE FERRARI2, GIANNA FABI1, VERA SALVALAGGIO1, ELISA PUNZO1, CLARA CUICCHI1, ANGELA SANTELLI1, ALESSIA CARIANI2, FAUSTO TINTI2, ANNA NORA TASSETTI1 and GIUSEPPE SCARCELLA1 1 Istituto di Scienze Marine (ISMAR), Consiglio Nazionale delle Ricerche (CNR), L.go Fiera della Pesca, 2, 60125 Ancona, Italy 2 Department of Biological, Geological & Environmental Sciences (BiGeA), University of Bologna, Via Selmi, 3, 40126, Bologna, Italy Corresponding author: [email protected] Handling Editor: Fabio Crocetta Received: 11 October 2016; Accepted: 3 August 2017; Published on line: 7 December 2017 Abstract Morphological analysis, molecular characterization, and a study of the distribution and density of Anadara kagoshimensis (Tokunaga, 1906) specimens collected in the Adriatic Sea were carried out using materials and data collected in the course of 329 bottom trawl hauls conducted in five yearly surveys, from 2010 to 2014. Morphological and molecular analysis allowed clarifying the confused taxonomy of the largest alien ark clam species invading Italian waters and the Mediterranean Sea. Analysis of the distribution and density data demonstrated that, along the Italian coast, A. kagoshimensis is mostly found at depths of 8 to 50 m, with a catch frequency of more than 98% in the hauls involving silty-clay sediment at a depth of 8-30 m. The hotspot map clearly shows a reduction in the distribution area of the species from 2010 to 2012. -

Quality Molluscan Genomes Jin Sun 1, Runsheng Li 2, Chong Chen 3, Julia D
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.31.424979; this version posted January 2, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Benchmarking Oxford Nanopore read assemblers for high- quality molluscan genomes Jin Sun 1, Runsheng Li 2, Chong Chen 3, Julia D. Sigwart 4,5, Kevin M. Kocot 6* 1 Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao 266003, China 2 Department of Infectious Diseases and Public Health, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Kowloon, Hong Kong, China 3 X-STAR, Japan Agency for Marine-Earth Science and Technology (JAMSTEC), 2-15 Natsushima-cho, Yokosuka, Kanagawa Prefecture 237-0061, Japan 4 Senckenberg Museum, Frankfurt, Germany 5 Queen’s University Belfast, Marine Laboratory, Portaferry, N Ireland 6 Department of Biological Sciences and Alabama Museum of Natural History, University of Alabama, Tuscaloosa, Alabama, 35487, USA Keywords: Molluscan genomes, assembly, Oxford Nanopore Technology, scaly-foot snail, Mytilus, phylogeny Summary Choosing the optimum assembly approach is essential to achieving a high-quality genome assembly suitable for comparative and evolutionary genomic investigations. Significant recent progress in long-read sequencing technologies such as PacBio and Oxford Nanopore Technologies (ONT) also brought about a large variety of assemblers. Although these have been extensively tested on model species such as Homo sapiens and Drosophila melanogaster, such benchmarking has not been done in Mollusca which lacks widely adopted model species. -

The First Record from Laos of Plotosus Canius (Teleostei: Plotosidae)
1 Ichthyological Exploration of Freshwaters/IEF-1167/pp. 1-8 Published 24 June 2021 LSID: http://zoobank.org/urn:lsid:zoobank.org:pub:3D2AC8FC-D4BC-4430-BAD5-F56074F5FCBC DOI: http://doi.org/10.23788/IEF-1167 The first record from Laos of Plotosus canius (Teleostei: Plotosidae) Kent G. Hortle* and Somphone Phommanivong** We record the first capture from Laos of the diadromous eel-tail catfish Plotosus canius as a single specimen of total length 1111 mm from Phapheng Channel, which is the most easterly anabranch of the Mekong in the Khone Falls system in southern Laos. The capture location is about 2.5 km downstream of Phapheng Waterfall and about 2.1 km upstream of the Cambodian border with Laos. As the location is about 720 km from the sea and at least 500 km upstream of any possible saline influence, this record shows that some large individuals of this diadromous species, which breeds in brackish waters, may penetrate long distances into freshwaters, where they feed on crabs and molluscs. Introduction nests and guard their fry, as described for another estuarine plotosid Cnidoglanis macrocephalus (Lau- Plotosus canius is an eel-tail catfish species wide- renson et al., 1993) and for a freshwater plotosid spread in the Indo-pacific Region from Sri Lanka Tandanus tandanus (Whitley, 1941). to New Guinea. It is found in estuaries and turbid Kottelat (2001) recorded 415 indigenous spe- coastal waters (Gomon, 1984), and in the ‘lower cies of fish from the Mekong River basin in Laos. parts’ of rivers, including the Mekong River sys- Among these he included Plotosus canius as tem in Cambodia and Vietnam (Rainboth, 1996); being: ‘tentatively recorded from Laos; its pres- however the upstream limit of its distribution in ence needs confirmation’.