Bivalvia, Veneridae) (Carpenter, 1864
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Lioconcha Castrensis Species Group (Bivalvia : Veneridae), with the Description of Two New Species
Molluscan Research 30(3): 117–124 ISSN 1323-5818 http://www.mapress.com/mr/ Magnolia Press The Lioconcha castrensis species group (Bivalvia : Veneridae), with the description of two new species SANCIA E.T. VAN DER MEIJ1, ROBERT G. MOOLENBEEK2 & HENK DEKKER2 1 Netherlands Centre for Biodiversity Naturalis (department of Marine Zoology), P.O. Box 9517, 2300 RA Leiden, The Nether- lands. Email: [email protected] (corresponding author) 2 Netherlands Centre for Biodiversity Naturalis (section Zoological Museum of Amsterdam), Mauritskade 57, 1092 AD Amsterdam, The Netherlands. Email: [email protected] Abstract Part of the genus Lioconcha Mörch, 1853 is reviewed. Species strongly resembling Lioconcha castrensis (Linnaeus, 1758) are discussed and two new species are described: Lioconcha arabaya n. sp. from the Northwest Indian Ocean and Lioconcha rumphii n. sp. from Thailand and Sumatra. These three species, together with Lioconcha macaulayi Lamprell & Healy, 2002, share many morphological similarities and we suspect them to be closely related. They are referred to as the Lioconcha cast- rensis species group. Furthermore, lectotypes of Venus castrensis Linnaeus, 1758, and Venus fulminea Röding, 1798, are desig- nated. The latter is considered a junior synonym of V. castrensis. Key words: Indo-Pacific, Mollusca, Persian Gulf, Red Sea, taxonomy Introduction between the anterior and posterior extremities, height is measured vertically from the umbo to the ventral margin and The delimitation within the tropical venerid genus Lioconcha total width (or inflation) is the greatest distance between the Mörch, 1853, is problematic, due to high levels of external surfaces of the paired valves. For an extensive list of intraspecific morphological variability and relatively few synonyms of figured specimens of Lioconcha castrensis we useful morphological characters (Lamprell and Healy 2002). -

Chec List Bivalves of the São Sebastião Channel, North Coast Of
Check List 10(1): 97–105, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Bivalves of the São Sebastião Channel, north coast of the PECIES S São Paulo State, Brazil OF Lenita de Freitas Tallarico 1*, Flávio Dias Passos 2, Fabrizio Marcondes Machado 3, Ariane Campos 1, ISTS 1 1,4 L Shirlei Maria Recco-Pimentel and Gisele Orlandi Introíni 1 Universidade Estadual de Campinas, Instituto de Biologia, Departamento de Biologia Estrutural e Funcional. R. Charles Darwin, s/n - Bloco N, Caixa Postal 6109. CEP 13083-863. Campinas, SP, Brazil. 2 Universidade Estadual de Campinas, Instituto de Biologia, Departamento de Biologia Animal. Rua Monteiro Lobato, 255, Caixa Postal 6109. CEP 13083-970. Campinas, SP, Brazil. 3 Programas de Pós-Graduação em Ecologia e Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas. R. Bertrand Russell, s/n, Caixa Postal 6109, CEP 13083-970. Campinas, SP, Brazil. 4 Universidade Federal de Ciências da Saúde de Porto Alegre, Departamento de Ciências Básicas da Saúde. R. Sarmento Leite, 245. CEP 90050-170. Porto Alegre, RS, Brazil. * Corresponding author. E-mail: [email protected] Abstract: The north coast of the São Paulo State, Brazil, presents great bivalve diversity, but knowledge about these organisms, especially species living subtidally, remains scarce. Based on collections made between 2010 and 2012, the present work provides a species list of bivalves inhabiting the intertidal and subtidal zones of the São Sebastião Channel. Altogether, 388 living specimens were collected, belonging to 52 species of 34 genera, grouped in 18 families. -

Faunistic Assemblages of a Sublittoral Coarse Sand Habitat of the Northwestern Mediterranean
Scientia Marina 75(1) March 2011, 189-196, Barcelona (Spain) ISSN: 0214-8358 doi: 10.3989/scimar.2011.75n1189 Faunistic assemblages of a sublittoral coarse sand habitat of the northwestern Mediterranean EVA PUBILL 1, PERE ABELLÓ 1, MONTSERRAT RAMÓN 2,1 and MARC BAETA 3 1 Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta, 37-49, 08003 Barcelona, Spain. E-mail: [email protected] 2 Instituto Español de Oceanografía, Centre Oceanogràfic de les Balears, Moll de Ponent s/n, 07015 Palma de Mallorca, Spain. 3 Tecnoambiente S.L., carrer Indústria, 550-552, 08918 Badalona, Spain. SUMMARY: The sublittoral megabenthic assemblages of a northwestern Mediterranean coarse sandy beach exploited for the bivalve Callista chione were studied. The spatial and bathymetric variability of its distinctive faunal assemblages was characterised by quantitative sampling performed with a clam dredge. The taxa studied were Mollusca Bivalvia and Gastropoda, Crustacea Decapoda, Echinodermata and Pisces, which accounted for over 99% of the total biomass. Three well- differentiated species assemblages were identified: (1) assemblage MSS (Medium Sand Shallow) in medium sand (D50=0.37 mm) and shallow waters (mean depth =6.5 m), (2) assemblage CSS (Coarse Sand Shallow) in coarse sand (D50=0.62 mm) in shallow waters (mean depth =6.7 m), and (3) assemblage CSD (Coarse Sand Deep) in coarse sand (D50=0.64 mm) in deeper waters (mean depth =16.2 m). Assemblage MSS was characterised by the codominance of the bivalves Mactra stultorum and Acanthocardia tuberculata. C. chione was dominant in both density and biomass in assemblages CSS and CSD. -

Geoducks—A Compendium
34, NUMBER 1 VOLUME JOURNAL OF SHELLFISH RESEARCH APRIL 2015 JOURNAL OF SHELLFISH RESEARCH Vol. 34, No. 1 APRIL 2015 JOURNAL OF SHELLFISH RESEARCH CONTENTS VOLUME 34, NUMBER 1 APRIL 2015 Geoducks — A compendium ...................................................................... 1 Brent Vadopalas and Jonathan P. Davis .......................................................................................... 3 Paul E. Gribben and Kevin G. Heasman Developing fisheries and aquaculture industries for Panopea zelandica in New Zealand ............................... 5 Ignacio Leyva-Valencia, Pedro Cruz-Hernandez, Sergio T. Alvarez-Castaneda,~ Delia I. Rojas-Posadas, Miguel M. Correa-Ramırez, Brent Vadopalas and Daniel B. Lluch-Cota Phylogeny and phylogeography of the geoduck Panopea (Bivalvia: Hiatellidae) ..................................... 11 J. Jesus Bautista-Romero, Sergio Scarry Gonzalez-Pel aez, Enrique Morales-Bojorquez, Jose Angel Hidalgo-de-la-Toba and Daniel Bernardo Lluch-Cota Sinusoidal function modeling applied to age validation of geoducks Panopea generosa and Panopea globosa ................. 21 Brent Vadopalas, Jonathan P. Davis and Carolyn S. Friedman Maturation, spawning, and fecundity of the farmed Pacific geoduck Panopea generosa in Puget Sound, Washington ............ 31 Bianca Arney, Wenshan Liu, Ian Forster, R. Scott McKinley and Christopher M. Pearce Temperature and food-ration optimization in the hatchery culture of juveniles of the Pacific geoduck Panopea generosa ......... 39 Alejandra Ferreira-Arrieta, Zaul Garcıa-Esquivel, Marco A. Gonzalez-G omez and Enrique Valenzuela-Espinoza Growth, survival, and feeding rates for the geoduck Panopea globosa during larval development ......................... 55 Sandra Tapia-Morales, Zaul Garcıa-Esquivel, Brent Vadopalas and Jonathan Davis Growth and burrowing rates of juvenile geoducks Panopea generosa and Panopea globosa under laboratory conditions .......... 63 Fabiola G. Arcos-Ortega, Santiago J. Sanchez Leon–Hing, Carmen Rodriguez-Jaramillo, Mario A. -

Foot Abnormalities in Venericardia Antiquata and Venus Verrucosa from the Bizerte Lagoon Complex
e Rese tur arc ul h c & a u D q e A v e f l o o Béjaoui et al., J Aquac Res Development 2016, 7:7 l p a m n Journal of Aquaculture r e u n DOI: 10.4172/2155-9546.1000434 o t J ISSN: 2155-9546 Research & Development Research Article OpenOpen Access Access Foot Abnormalities in Venericardia antiquata and Venus verrucosa from the Bizerte Lagoon Complex (Northern Tunisia): Hydrodynamics and Sediment Texture Inductions Jihen Maâtoug Béjaoui, Ferdaous Jaafar Kefi, Anwar Mleiki and Najoua Trigui El Menif* Faculty of Sciences of Bizerte (FSB), Laboratory of Environment Biomonitoring, University of Carthage, Bizerte, Tunisia Abstract The Examination of the soft part of the two bivalve species Venericardia antiquata (Linnaeus 1758) and Venus verrucosa (Linnaeus 1758), that occur together in northern coast of Tunisia, allowed us to discover for the first time the presence of morphological abnormalities affecting the foot of many individuals (annual rate of 31.6%). The presence of a developed byssus was also detected in some specimens of V. antiquata. A classification scale of this malformation, established depending on the degree of this anomaly, showed six initial types that evolve to form two or three feet, at the posterior and/or anterior sides of the animal. In order to determine the causes of this malformation, experiments of transplantation were carried out. Specimens of V. verrucosa collected from Zarzouna station were transplanted in Chaâra station which is characterized by low rate of malformations, low hydrodynamics and different sediment type and vice versa. Results revealed that foot malformations degree is highly correlated with both hydrodynamics and substrate type. -

Shell Classification – Using Family Plates
Shell Classification USING FAMILY PLATES YEAR SEVEN STUDENTS Introduction In the following activity you and your class can use the same techniques as Queensland Museum The Queensland Museum Network has about scientists to classify organisms. 2.5 million biological specimens, and these items form the Biodiversity collections. Most specimens are from Activity: Identifying Queensland shells by family. Queensland’s terrestrial and marine provinces, but These 20 plates show common Queensland shells some are from adjacent Indo-Pacific regions. A smaller from 38 different families, and can be used for a range number of exotic species have also been acquired for of activities both in and outside the classroom. comparative purposes. The collection steadily grows Possible uses of this resource include: as our inventory of the region’s natural resources becomes more comprehensive. • students finding shells and identifying what family they belong to This collection helps scientists: • students determining what features shells in each • identify and name species family share • understand biodiversity in Australia and around • students comparing families to see how they differ. the world All shells shown on the following plates are from the • study evolution, connectivity and dispersal Queensland Museum Biodiversity Collection. throughout the Indo-Pacific • keep track of invasive and exotic species. Many of the scientists who work at the Museum specialise in taxonomy, the science of describing and naming species. In fact, Queensland Museum scientists -

Genetic Relationship of Asiatic Hard Clam Populations Collected in Northern Coastal Provinces in Vietnam Based on Mtdna Sequence Analysis
Journal of Aquaculture & Marine Biology Genetic relationship of asiatic hard clam populations collected in northern coastal provinces in Vietnam based on mtDNA sequence analysis Abstract Research Article The genetic relationship of some Asiatic hard clam (Meretrix meretrix) based on mtDNA Volume 7 Issue 1 - 2018 COI sequence analysis was investigated for populations collected in Thai Binh, Nam Dinh, Nghe An provinces in Vietnam. In addition, this research also targets at species Vu Thi Trang,1 Le Thi Quynh Chi,3 Chu Chi identification based on COI sequences. In total of 59 sequences analyzed, 19 sequences Thiet,2 Nguyen Huu Duc,3 Tran Thi Thuy Ha1 belonged to Meretrix meretrix species with Gen Bank accession number DQ399399.1. 17 1Centre of Aquaculture Biotechnology, Research Institute for sequences of M. meretrix were used for genetic relationship analysis among 3 populations. Aquaculture No.1, Vietnam In which, 6 polymorphic sites, 3 parsimony informative sites and 4 haplotypes observed 2Aquaculture Research Sub-Institute for North Central for the COI gene. Moderately genetic population diversity was observed, overall haplotype (ARSINC), Research Institute for Aquaculture No.1, Vietnam and nucleotide diversity were 0.476±0.233 and 0.00151±0.00069, respectively. Generally, 3Faculty of Biotechnology, Vietnam National University of Agriculture, Vietnam genetic differentiation (FST) (FST < 0.15) was moderate. The genetic distance was rather low, which ranged from 0.001 (Thai Binh–NgheAn, Thai Binh–Nam Dinh populations) to 0.002 (Nam Dinh – Nghe An populations). The result of haplotype network constructing Correspondence: Vu Thi Trang, Centre of Aquaculture indicated that populations shared common haplotype and there was no specific isolation Biotechnology, Research Institute for Aquaculture No.1, Vietnam, of the haplotypes of the populations. -

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -

Functional Traits of a Native and an Invasive Clam of the Genus Ruditapes Occurring in Sympatry in a Coastal Lagoon
www.nature.com/scientificreports OPEN Functional traits of a native and an invasive clam of the genus Ruditapes occurring in sympatry Received: 19 June 2018 Accepted: 8 October 2018 in a coastal lagoon Published: xx xx xxxx Marta Lobão Lopes1, Joana Patrício Rodrigues1, Daniel Crespo2, Marina Dolbeth1,3, Ricardo Calado1 & Ana Isabel Lillebø1 The main objective of this study was to evaluate the functional traits regarding bioturbation activity and its infuence in the nutrient cycling of the native clam species Ruditapes decussatus and the invasive species Ruditapes philippinarum in Ria de Aveiro lagoon. Presently, these species live in sympatry and the impact of the invasive species was evaluated under controlled microcosmos setting, through combined/manipulated ratios of both species, including monospecifc scenarios and a control without bivalves. Bioturbation intensity was measured by maximum, median and mean mix depth of particle redistribution, as well as by Surface Boundary Roughness (SBR), using time-lapse fuorescent sediment profle imaging (f-SPI) analysis, through the use of luminophores. Water nutrient concentrations (NH4- N, NOx-N and PO4-P) were also evaluated. This study showed that there were no signifcant diferences in the maximum, median and mean mix depth of particle redistribution, SBR and water nutrient concentrations between the diferent ratios of clam species tested. Signifcant diferences were only recorded between the control treatment (no bivalves) and those with bivalves. Thus, according to the present work, in a scenario of potential replacement of the native species by the invasive species, no signifcant diferences are anticipated in short- and long-term regarding the tested functional traits. -

Mollusca: Veneridae) in the Western Pacific Ocean1
Genetic Relationships among Species of Meretrix (Mollusca: Veneridae) in the Western Pacific Ocean1 Ayako Yashiki Yamakawa,2,3,6 Masashi Yamaguchi,4,5 and Hideyuki Imai4 Abstract: We compared allozymes at 12 loci in 12 populations of six species of Meretrix: M. lusoria ( Japan, Korea, and Taiwan), M. petechialis (China and Ko- rea), M. ovum (Thailand and Mozambique), M. lyrata (China), M. lamarckii ( Ja- pan), and Meretrix sp. A (Okinawa, Japan). Our allozyme results were generally consistent with the major groupings currently recognized within the genus based on morphological characters. However, we found two cryptic or un- described species: Meretrix sp. A from Okinawa and M. cf. lusoria from Taiwan. The shell characters of Meretrix sp. A were similar to those of M. lamarckii, but the species was genetically distinct (Nei’s genetic distance D > 0.845) from all other species examined. The Taiwanese Meretrix population was morphologi- cally indistinguishable from Japanese M. lusoria, although the genetic distance between the Taiwanese and Japanese populations showed a high degree of ge- netic differentiation (D > 0.386). Meretrix lusoria seedlings were introduced into Taiwan from Japan in the 1920s, and Japanese M. lusoria was previously thought to be established as a cultured stock. However, our results suggest that the Taiwanese population may represent a sibling or cryptic species of M. lusoria. Asianhardclams, genus Meretrix (Vener- (Yoosukh and Matsukuma 2001). These idae), are commercially important bivalves clams inhabit the tidal flats, estuaries, and in East and Southeast Asia and East Africa sandy beaches of the Indian Ocean, including East Africa and Southeast Asia, and the west- ern Pacific along the Chinese coast, Korean 1 Financial support was provided from the 21st Peninsula, and Japanese Archipelago. -
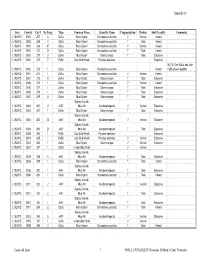
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

Induccion Al Desove Y Desarrollo Larval Del Molusco Bivalvo Chione Cancellata
Induccion al Desove y Desarrollo Larval del Molusco Bivalvo Chione cancellata JOSE RENGEL1, LUGO GUELMELIT2, LUIS TORRES2, y CARL HOLUIS MARIN 1Universidad Nacional Experimental Francisco De Complejo Docente El Sabino, Prolongacion Tachira, Sector Universita- rio Punto Fijo, Falcon 4102 Venezuela. 2Universidad Nacional Experimental Francisco De Miranda, Programa De Ing. Pesquera Complejo Docente El Sabino, Prolongacion Tachira, Sector Universidad, Punto Fijo, Falcon 4102 Venezuela RESUMEN El guacuco, Chione cancellata, es una de las especies de moluscos bivalvos de mayor importancia comercial en la costas de la Bahía de Amuay. La mayoría de los pobladores de la zona, viven de su extracción y comercialización. Hasta el momento no se tienen información sobre el desarrollo larval de esta especie, para ser explotado en un futuro cultivo. Por tal motivo, se realizó la inducción al desove de este molusco, utilizando como técnica el choque térmico y descripción de su desarrollo larval. Se obtuvo con éxito el desove después de tres horas de tratamiento térmico y los embriones obtenidos, fueron colocadas y mantenidas en recipientes de 18 L con 15 L de agua de mar filtrada y esterilizadas a 35 UPS y temperatura promedio de 27 ºC, con recambio del 100 % del agua, cada 24 horas. Las larvas se alimentaron con la microalgas Chaetoceros calcitrans, Nannocloropsis sp., y Tretaselmis sp a una concentración de 20.000 cel./ml, de cada una. El desarrollo embrionario del Chione sp. se generó con toda normalidad, alcanzando todas sus fases larvales de la siguiente