Dislocated Tongue Muscle Attachment Connected to Cleft Palate Formation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Appearance of Foramen in the Internal Aspect of the Mental
Okajimas Folia Anat. Jpn., 82(3): 83–88, November, 2005 The Appearance of Foramen in the Internal Aspect of the Mental Region of Mandible from Japanese Cadavers and Dry Skulls Under Macroscopic Observation and Three-dimensional CT Images By Shunji YOSHIDA1),TaisukeKAWAI2),KoichiroOKUTSU2), Takashi YOSUE2), Hitoshi TAKAMORI3), Masataka SUNOHARA and Iwao SATO1) 1Department of Anatomy, 2Department of Oral and Maxillofacial Radiology School of Dentistry at Tokyo, 3Oral Implant Clinic, Nippon Dental University at Tokyo, Tokyo, Japan – Received for Publication, June 28, 2005 – Key Words: Lingual foramen, CT, Mandible Summary: The lingual canal with foramen displays different appearances on the internal surfaces of mandible as con- firmed by macroscopic observation and computerized tomography (CT). The lingual canal was observed in the inside of mental region run to the outside of lingual foramen, which is extend internally from mandibular canal in right and left sides of the mandible in cadavers (13 sides out of 88 sides) and in dry skulls (43 out of 94 sides) examined. The spinal foramen connected with mental canal occurred at the midline of mandible in 6 cases (6 out of 47 cases) in dry skulls. In this small foramen, the inferior alveolar artery give some branches to the inside of mental region at the anterior man- dible and which may be run pass through the lingual canal to the lingual foramen, where they emerge to enter the mylohyoid or anterior belly of digastric muscles. The observations of these are important considerations for surgical placement of dental implants in the region in the mandible. The anatomical location, course and arrange- treatments. -

Mechanics in the Production of Mandibular Technique. I
Mechanics in the Production of Mandibular Fractures: A Study with the "Stresscoat' Technique. I. Symphyseal Impacts DONALD F. HUELKE Department of Anatomy, University of Michigan Medical School Ann Arbor, Michigan A recent study of 319 case histories of mandibular fractures by Hagan and Huelke' has shown that certain areas of the jaw are fractured more often than others and that the incidence of certain mandibular fractures is greater when the blow is directed to specific regions of the jaw. Relatively little is known about the response of the mandi- ble to impact, except that, when the magnitude of a blow is sufficient, the bone will break. How does the mandible fracture, and what are the mechanisms involved? These questions have not been answered because of the lack of experimental data. Obtaining these data is an engineering problem involving stresses, strains, impacts, energies, and forces, and thus engineering techniques must be used. This report, the first of a series of studies on the mechanism of mandibular frac- tures, presents data on forces and impacts applied to the chin point of the mandible and the resultant deformations of the bone. The results of these tests are correlated with certain clinical findings. Terminology.-Throughout this report certain terminology generally used in engi- neering will be employed. Some of these terms need to be defined. The term force is defined as a push or pull. The various types of force are illustrated in Figure 1. Ten- sile forces (tension) tend to pull an object apart; compressive forces (compression) push the particles forming an object together, while shearing forces make one part of an object slide over another part of the same object. -

Download The
Review of the arterial anatomy in the anterior mandible Review of the arterial vascular anatomy for implant placement in the anterior mandible Abstract Objective José Carlos Balaguer Marti,* Juan Guarinos,† The placement of implants in the anterior region of the mandible is not Pedro Serrano Sánchez,† Amparo Ruiz Torner,* free of risk and can even sometimes be life-threatening. The aim of this * * David Peñarrocha Oltra & Miguel Peñarrocha Diago article is to review the anatomy of the anterior mandible regarding the *Department of Stomatology, Faculty of Medicine and placement of implants in this region. Odontology, University of Valencia, Valencia, Spain † Department of Anatomy, Faculty of Medicine and Materials and methods Odontology, University of Valencia, Valencia, Spain An anatomical study was conducted in cadavers to analyze the various Corresponding author: anatomical structures of the anterior region of the mandible. A literature review was also undertaken. Dr. David Peñarrocha Oltra Clínicas odontológicas Gascó Oliag, 1 46021 Valencia Results Spain The sublingual and submental arteries are the main supply of the sublin- T & F +34 963 86 4139 [email protected] gual region. These arteries are usually located at a safe distance from the alveolar ridge, but in cases of severe atrophy or anatomical variations, there may be an increased risk of damage during the placement of dental How to cite this article: implants and serious complications may arise. Balaguer Marti JC, Guarinos J, Serrano Sánchez P, Ruiz Torner A, Peñarrocha Oltra D, Peñarrocha Diago M. Conclusion Review of the arterial vascular anatomy for implant placement in the anterior mandible. The injury of the vessels in the floor of the mouth could lead to severe complications. -

Chapter 2 Implants and Oral Anatomy
Chapter 2 Implants and oral anatomy Associate Professor of Maxillofacial Anatomy Section, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University Tatsuo Terashima In recent years, the development of new materials and improvements in the operative methods used for implants have led to remarkable progress in the field of dental surgery. These methods have been applied widely in clinical practice. The development of computerized medical imaging technologies such as X-ray computed tomography have allowed detailed 3D-analysis of medical conditions, resulting in a dramatic improvement in the success rates of operative intervention. For treatment with a dental implant to be successful, it is however critical to have full knowledge and understanding of the fundamental anatomical structures of the oral and maxillofacial regions. In addition, it is necessary to understand variations in the topographic and anatomical structures among individuals, with age, and with pathological conditions. This chapter will discuss the basic structure of the oral cavity in relation to implant treatment. I. Osteology of the oral area The oral cavity is composed of the maxilla that is in contact with the cranial bone, palatine bone, the mobile mandible, and the hyoid bone. The maxilla and the palatine bones articulate with the cranial bone. The mandible articulates with the temporal bone through the temporomandibular joint (TMJ). The hyoid bone is suspended from the cranium and the mandible by the suprahyoid and infrahyoid muscles. The formation of the basis of the oral cavity by these bones and the associated muscles makes it possible for the oral cavity to perform its various functions. -
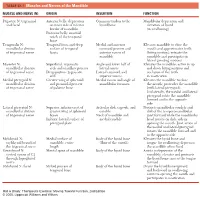
1 TABLE 23-1 Muscles and Nerves of the Mandible
0350 ch 23-Tab 10/12/04 12:19 PM Page 1 Chapter 23: The Temporomandibular Joint 1 TABLE 23-1 Muscles and Nerves of the Mandible MUSCLE AND NERVE (N) ORIGIN INSERTION FUNCTION Digastric N: trigeminal Anterior belly: depression Common tendon to the Mandibular depression and and facial on inner side of inferior hyoid bone elevation of hyoid border of mandible (in swallowing) Posterior belly: mastoid notch of the temporal bone Temporalis N: Temporal fossa and deep Medial and anterior Elevates mandible to close the mandibular division surface of temporal coronoid process and mouth and approximates teeth of trigeminal nerve fascia anterior ramus of (biting motion); retracts the mandible mandible and participates in lateral grinding motions Masseter N: Superficial: zygomatic Angle and lower half of Elevates the mandible; active in up mandibular division arch and maxillary process lateral ramus and down biting motions and of trigeminal nerve Deep portion: zygomatic Lateral coronoid and occlusion of the teeth arch superior ramus in mastication Medial pterygoid N: Greater wing of sphenoid Medial ramus and angle of Elevates the mandible to close mandibular division and pyramidal process mandibular foramen the mouth; protrudes the mandible of trigeminal nerve of palatine bone (with lateral pterygoid). Unilaterally, the medial and lateral pterygoid rotate the mandible forward and to the opposite side Lateral pterygoid N: Superior: inferior crest of Articular disk, capsule, and Protracts mandibular condyle and mandibular division greater wing of sphenoid condyle disk of the temporomandibular of trigeminal nerve bones Neck of mandible and joint forward while the mandibular Inferior: lateral surface of medial condyle head rotates on disk; aids in pterygoid plate opening the mouth. -

Smithsonian Miscellaneous Collections
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 121, NUMBER 8 WESTERN ATLANTIC SCORPIONFISHES BY ISAAC GINSBURd U. S. Fish and Wildlife Service -f'SS^af-o m (Publication 4106) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION MAY 28, 1953 SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 121, NUMBER 8 WESTERN ATLANTIC SCORPIONFISHES BY ISAAC GINSBURG U. S. Fish and Wildlife Service (Publication 4106) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION MAY 28, 1953 2^^e Bovi (§&itimovi (preec BALTIMORE, MS., n. S. A. WESTERN ATLANTIC SCORPIONFISHES By ISAAC GINSBURG U. S. Fish and Wildlife Service INTRODUCTION The present state of the taxonomy of the western Atlantic scor- paenids leaves much to be desired. The literature is filled with inade- quate original or supplementary descriptions of species, containing many unessential details of little or no practical use for the correct determination of the species, while the critical characters that dis- tinguish the species often are not considered adequately. I found it well-nigh impossible to identify and distinguish the species properly by the use of current accounts. One of the main factors that operate to bedevil the taxonomist who conscientiously tries to identify his specimens is the undue multiplication by past authors of the number of fictitious species. Such untenable "species" have been established, in large measure, as a result of failure to elaborate properly, or even to take into consideration, the intraspecific range of variability of taxonomic characters, or their change with growth which, in the scorpaenids, is considerable. The multiplication of names that have been proposed for scorpaenid species that have no existence in fact is as confusing as the grouping of two or more closely related species under one name that has entered into the literature of other families. -

A Review on Variations in Lingual Foramina of the Mandible Midline
Journal of Critical Reviews ISSN- 2394-5125 Vol 7, Issue 14, 2020 A REVIEW ON VARIATIONS IN LINGUAL FORAMINA OF THE MANDIBLE MIDLINE 1Jones Jayabalan, 2M.R.Muthusekhar 1Post Graduate Student Department of Oral and Maxillofacial Surgery Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India. 2Program Director Department of Oral and Maxillofacial Surgery Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India. Corresponding Author: Jones Jayabalan Post Graduate Student Department of Oral and Maxillofacial Surgery Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai-600077Tamil Nadu, India. Email id: [email protected] Received:16.04.2020 Revised: 21.05.2020 Accepted: 20.06.2020 Abstract The sublingual, submental arteries or their anastomosis perforate the lingual cortical plate through the lingual foramen. Both the arteries are branch of facial and lingual arteries respectively which either arises independently from the external carotid artery or arises from a common lingual facial trunk. The anterior mandibular midline at which the lingual foramen is frequently present is subjected to various procedure like dental implants, genioplasty, tori removal, block graft harvesting, screwing with or without plating following trauma or osteotomy. There is a wide range of anatomical variations of lingual foramen among individuals. Cone-beam computed tomography (CBCT) has been shown to be superior to panoramic radiographs in displaying mandibular lingual foramen and its variations. Numerous studies have been carried out to examine the frequency, diameter, and other anatomical features of the lingual foramen and its canals. -

1 TERMINOLOGIA ANTHROPOLOGICA Names of The
TERMINOLOGIA ANTHROPOLOGICA Names of the parts of the human body, terms of aspects and relationships, and osteological terminology are as in Terminologia Anatomica. GENERAL TERMS EXPLANANTION ADAPTATION Adjustment and change of an organism to a specific environment, due primarily to natural selection. ADAPTIVE RADIATION Divergence of an ancestral population through adaption and speciation into a number of ecological niches. ADULT Fully developed and mature individual ANAGENESIS The progressive adaption of a single evolutionary line, where the population becomes increasingly specialized to a niche that has remained fairly constant through time. ANCESTRY One’s family or ethnic descent, the evolutionary or genetic line of descent of an animal or plant / Ancestral descent or lineage ANTEMORTEM Biological processes that can result in skeletal modifications before death ANTHROPOCENTRICISM The belief that humans are the most important elements in the universe. ANTHROPOLOGY The study of human biology and behavior in the present and in the past ANTHROPOLOGIST BIOLOGICAL A specialist in the subfield of anthropology that studies humans as a biological species FORENSIC A specialist in the use of anatomical structures and physical characteristics to identify a subject for legal purposes PHYSICAL A specialist in the subfield of anthropology dealing with evolutionary changes in the human bodily structure and the classification of modern races 1 SOCIAL A specialist in the subfield of anthropology that deals with cultural and social phenomena such as kingship systems or beliefs ANTHROPOMETRY The study of human body measurement for use in anthropological classification and comparison ARCHETYPE That which is taken as the blueprint for a species or higher taxonomic category ARTIFACT remains of past human activity. -

Splanchnocranium
splanchnocranium - Consists of part of skull that is derived from branchial arches - The facial bones are the bones of the anterior and lower human skull Bones Ethmoid bone Inferior nasal concha Lacrimal bone Maxilla Nasal bone Palatine bone Vomer Zygomatic bone Mandible Ethmoid bone The ethmoid is a single bone, which makes a significant contribution to the middle third of the face. It is located between the lateral wall of the nose and the medial wall of the orbit and forms parts of the nasal septum, roof and lateral wall of the nose, and a considerable part of the medial wall of the orbital cavity. In addition, the ethmoid makes a small contribution to the floor of the anterior cranial fossa. The ethmoid bone can be divided into four parts, the perpendicular plate, the cribriform plate and two ethmoidal labyrinths. Important landmarks include: • Perpendicular plate • Cribriform plate • Crista galli. • Ala. • Ethmoid labyrinths • Medial (nasal) surface. • Orbital plate. • Superior nasal concha. • Middle nasal concha. • Anterior ethmoidal air cells. • Middle ethmoidal air cells. • Posterior ethmoidal air cells. Attachments The falx cerebri (slide) attaches to the posterior border of the crista galli. lamina cribrosa 1 crista galli 2 lamina perpendicularis 3 labyrinthi ethmoidales 4 cellulae ethmoidales anteriores et posteriores 5 lamina orbitalis 6 concha nasalis media 7 processus uncinatus 8 Inferior nasal concha Each inferior nasal concha consists of a curved plate of bone attached to the lateral wall of the nasal cavity. Each consists of inferior and superior borders, medial and lateral surfaces, and anterior and posterior ends. The superior border serves to attach the bone to the lateral wall of the nose, articulating with four different bones. -

Submandibular Region
20/02/2013 Learning Outcomes • The Mandible • The Submandibular – Surface Anatomy Region – Muscle Attachments – Submandibular Gland Submandibular Region • The Floor of the Mouth – Sublingual Gland (FOM) – Lingual Nerve – Muscles of the FOM • The Head & Neck Mohammed A Al-Muharraqi MBChB, BDS, MSc, MRCS Glas, FFD RCS Irel, MFDS RCS Eng • The Tongue Parasympathetics – Muscles of the Tongue e-mail: [email protected] Mandible Mandible Head of Condylar Process Coronoid Process Neck of Pterygoid Fovea Neck of Condylar Condylar Process Condylar Process Ramus of Mandible Process Condylar Process Anterior Border of Ramus Lingula Coronoid Process Mandibular Coronoid Lingual (Genial) Foramen (Mandibular) Foramen Notch Alveolar Ridge Mylohyoid Line Oblique Line Alveolar Ridge Posterior Border of Ramus of Mandible Ramus Angle of Mandible Incisive Fossa Mylohyoid Groove Angle of Mandible Symphysis Menti (Median Ridge) Inferior Border of Submandibular Fossa Body of Mandible Body Mental Protuberance Sublingual Fossa Digastric Fossa Mental Foramen Superior Mental Spine Inferior Mental Spine Mental Tubercle (Genial Tubercle) (Genial Tubercle) Inferior Border of Body Muscle Attachments The mental tubercle (a raised prominence at Mandible Ligaments the mental symphysis) is a point of muscular attachment. The external surface of the ramus is covered by the attachment of the masseter muscle. On the inner surface of the body of the mandible, there is a horizontal mylohyoid line, which attaches the mylohyoid muscle. Above it, there is a shallow depression for the sublingual salivary gland and below it a deeper depression for the submandibular gland. At the anterior ends of the mylohyoid lines and superior to them, near the symphysis, there is the genial tubercles. -

New Terminologia Anatomica: Cranium and Extracranial Bones of the Head P.P
Folia Morphol. Vol. 80, No. 3, pp. 477–486 DOI: 10.5603/FM.a2019.0129 R E V I E W A R T I C L E Copyright © 2021 Via Medica ISSN 0015–5659 eISSN 1644–3284 journals.viamedica.pl New Terminologia Anatomica: cranium and extracranial bones of the head P.P. Chmielewski Division of Anatomy, Department of Human Morphology and Embryology, Faculty of Medicine, Wroclaw Medical University, Wroclaw, Poland [Received: 12 October 2019; Accepted: 17 November 2019; Early publication date: 3 December 2019] In 2019, the updated and extended version of Terminologia Anatomica was published by the Federative International Programme for Anatomical Terminology (FIPAT). This new edition uses more precise and adequate anatomical names compared to its predecessors. Nevertheless, numerous terms have been modified, which poses a challenge to those who prefer traditional anatomical names, i.e. medical students, teachers, clinicians and their instructors. Therefore, there is a need to popularise this new edition of terminology and explain these recent changes. The anatomy of the head, including the cranium, the extracranial bones of the head, the soft parts of the face and the encephalon, poses a particular challenge for medical students but also engenders enthusiasm in those of them who are astute learners. The new version of anatomical terminology concerning the human skull (FIPAT 2019) is presented and briefly discussed in this synopsis. The aim of this article is to present, popularise and explain these interesting modifications that have recently been endorsed by the FIPAT. Based on teaching experience at the Division of Anatomy/Department of Anatomy at Wroclaw Medical University, a brief description of the human skull is given here. -

Facial Skeleton. Orbit and Nasal Cavity
Facial skeleton. Orbit and nasal cavity. Sándor Katz M.D.,Ph.D. Skull Cerebrocranium= Viscerocranium= Neurocranium Facial skeleton • Frontal bone • Nasal bone • Sphenoid bone • Lacrimal bone • Temporal bone • Ethmoid bone • Parietal bone • Maxilla • Occipital bone • Mandible • Zygomatic bone • Vomer • Palatine bone • Inferior nasal concha • Hyoid bone Viscerocranium= Facial skeleton • Nasal bone • Lacrimal bone • Ethmoid bone • Maxilla • Mandible • Zygomatic bone • Vomer • Palatine bone • Inferior nasal concha • Hyoid bone Nasal bone • internasal septum • piriform aperture Lacrimal bone • posterior lacrimal crest • lacrimal groove • nasolacrimal canal • lacrimal sac Ethmoid bone: perpendicularular plate • crista galli Ethmoid bone: cribriform plate • foramina cribrosa Ethmoid bone: cribriform plate • ethmoidal air cells • ethmoidal labyrinth • orbital (lateral) plate • superior and middle nasal conchae Ethmoid bone: cribriform plate • ethmoid bulla (8) • uncinate process • semilunar hiatus Maxilla: body • infraorbital groove • infraorbital canal • infraorbital foramen • infraorbital margin Maxilla: body • canine fossa Maxilla: body • tuber maxillae • pterygomaxillary fissure • maxillary sinus • maxillary hiatus Maxilla: frontal process • aterior lacrimal crest • piriform aperture zygomatic process Maxilla: alveolar process • alveolar arch • alveolar yokes • anterior nasal spine Maxilla: alveolar process • dental alveolae • interalveolar septa • interradicular septa Maxilla: palatine process • incisive canal • median palatine suture • transverse