Homothallic Mating
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Genetic Systems in Filamentous Ascomycetes
Evolution of Genetic Systems in Filamentous Ascomycetes Evolutie van genetische systemen in hyphenvormende zakjeszwammen 0000 0513 3836 Promotor: dr. R.F. Hoekstra hoogleraar in de populatie- en kwantitatieve genetica fjtfoiißi f ßin Maarten J. Nauta Evolution of Genetic Systems in Filamentous Ascomycetes Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. C.M. Karssen, in het openbaar te verdedigen op woensdag 12januar i 1994 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen. 15 0 S(p^ZJ> These investigations were supported by the Netherlands Organization for Scientific Research (N.W.O.). BibUt/FHEEK LAMDbOirWUNIVERSITEJi. WAGE NINGE N CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Nauta, Maarten J. Evolution of genetic systems in filamentous ascomycetes / Maarten J. Nauta. - [ S.l. : s.n.]. -111 . Thesis Wageningen. - With ref. - With summary in Dutch. ISBN 90-5485-199-6 Subject headings: population genetics / ascomycetes. omslagontwerp: Ernst van Cleef foto omslag: Barrages tussen verschillende stammen van Podospora anserina als gevolg van vegetatieve incompatibiliteit. (met dank aan Inge Haspels) aan mijn ouders Voorwoord Dit proefschrift is het resultaat van vier jaar onderzoek, verricht bij de vakgroep Erfelijkheidsleer van de Landbouwuniversiteit in Wageningen. In zekere zin valt zo'n proefschrift te vergelijken met een levend wezen. Uit de genetica is bekend dat de verschijningsvorm van elk levend wezen tot stand komt door een combinatie van erfelijke aanleg en invloeden uit de omgeving. Voor een proefschrift geldt eigenlijk hetzelfde: Zowel het werk van de auteur, als de bijdragen van zijn omgeving zijn onontbeerlijk om tot een verschijningsvorm te komen. -

Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho
Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho To cite this version: James Umen, Susana Coelho. Algal Sex Determination and the Evolution of Anisogamy. Annual Review of Microbiology, Annual Reviews, 2019, 73 (1), 10.1146/annurev-micro-020518-120011. hal- 02187088 HAL Id: hal-02187088 https://hal.sorbonne-universite.fr/hal-02187088 Submitted on 17 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Microbiol. 2019. 73:X–X https://doi.org/10.1146/annurev-micro-020518-120011 Copyright © 2019 by Annual Reviews. All rights reserved Umen • Coelho www.annualreviews.org • Algal Sexes and Mating Systems Algal Sex Determination and the Evolution of Anisogamy James Umen1 and Susana Coelho2 1Donald Danforth Plant Science Center, St. Louis, Missouri 63132, USA; email: [email protected] 2Sorbonne Université, UPMC Université Paris 06, CNRS, Algal Genetics Group, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, F-29688, Roscoff, France [**AU: Please write the entire affiliation in French or write it all in English, rather than a combination of English and French**] ; email: [email protected] Abstract Algae are photosynthetic eukaryotes whose taxonomic breadth covers a range of life histories, degrees of cellular and developmental complexity, and diverse patterns of sexual reproduction. -

Sex in the Extremes: Lichen-Forming Fungi
Mycologist, Volume 19, Part 2 May 2005. ©Cambridge University Press Printed in the United Kingdom. DOI: 10.1017/S0269915XO5002016 Sex in the extremes: lichen-forming fungi FABIAN A. SEYMOUR, PETER D. CRITTENDEN & PAUL S. DYER* School of Biology, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. Tel. +44 (0) 115 9513203, Fax +44 (0) 115 9513251 E-mail: [email protected]; [email protected] *Corresponding Author Lichens are characteristically found in environments subject to extremes of temperature, desiccation and low nutrient status. Despite this sexual structures are often formed in abundance. The underlying mechanisms of sex in lichen-forming fungi are discussed, together with possible ecological reasons for the persistence of sexuality. Special features of lichen sex are highlighted including sex at the limits of life on earth in Antarctica, re-licheniza- tion following sex and dispersal, and the perennial nature of lichen fruiting bodies. Keywords: lichen, fungi, sex, breeding system, (98%) belonging to the Ascomycotina (Kirk et al., symbiosis, extreme environments, Antarctica 2001). They display a variety of morphologies, from flattened crust (crustose) or leafy (foliose) forms to Lichens - living together in a long-term relation- shrubby or pendulous fruticose types (Honegger, 2001) ship (Figs 3, 4, 7, 8). Lichens are seen as a textbook example of a successful Life in extreme environments mutualistic symbiosis. They consist of at least two A key characteristic of lichens is that they have a organisms: a fungus (the ‘mycobiont’), and an remarkable ability to tolerate extreme environmental intimately associated photosynthetic partner (the conditions and sustain growth despite frequent cycles ‘photobiont’). -

Parasex Generates Phenotypic Diversity De Novo and Impacts Drug Resistance and Virulence in Candida Albicans
| INVESTIGATION Parasex Generates Phenotypic Diversity de Novo and Impacts Drug Resistance and Virulence in Candida albicans Matthew P. Hirakawa, Darius E. Chyou,1 Denis Huang,1 Aaron R. Slan,1 and Richard J. Bennett2 Department of Molecular Microbiology and Immunology, Brown University, Providence, Rhode Island 02912 ORCID ID: 0000-0001-7563-484X (R.J.B.) ABSTRACT Candida albicans is a diploid fungus that is a frequent cause of mucosal and systemic infections in humans. This species exhibits an unusual parasexual cycle in which mating produces tetraploid cells that undergo a nonmeiotic program of concerted chromosome loss to return to a diploid or aneuploid state. In this work, we used a multipronged approach to examine the capacity of parasex to generate diversity in C. albicans. First, we compared the phenotypic properties of 32 genotyped progeny and observed wide-ranging differences in fitness, filamentation, biofilm formation, and virulence. Strikingly, one parasexual isolate displayed in- creased virulence relative to parental strains using a Galleria mellonella model of infection, establishing that parasex has the potential to enhance pathogenic traits. Next, we examined parasexual progeny derived from homothallic, same-sex mating events, and reveal that parasex can generate diversity de novo from identical parental strains. Finally, we generated pools of parasexual progeny and examined resistance of these pools to environmental stresses. Parasexual progeny were generally less fit than control strains across most test conditions, but showed an increased ability to grow in the presence of the antifungal drug fluconazole (FL). FL-resistant progeny were aneuploid isolates, often being diploid strains trisomic for both Chr3 and Chr6. -
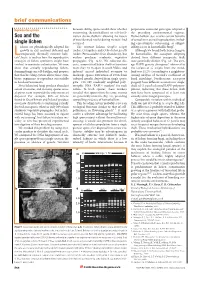
Brief Comms Layout 6/4Mx
brief communications Reproductive systems between sibling spores would show whether perpetuates successful genotypes adapted to outcrossing (heterothallism) or self-fertil- the prevailing environmental regimes. Sex and the ization (homothallism: allowing the fusion Homothallism also retains certain benefits of two identical nuclei during meiosis) had of sexual over asexual reproduction, includ- single lichen occurred. ing opportunistic outcrossing, as obligate ichens are physiologically adapted for The crustose lichens Graphis scripta selfing is rare in homothallic fungi8. growth in dry, nutrient-deficient and (order: Ostropales) and Ochrolechia parella Although we found both lichen fungi to Ltemporarily thermally extreme habi- (order: Pertusariales) fruit abundantly, but be homothallic, the ascospore offspring tats1, but it is unclear how the reproductive neither produces symbiotic vegetative derived from different conspecific thalli strategies of lichen symbionts might have propagules (Fig. 1a,b). We collected dis- were genetically distinct (Fig. 1d). The aver- evolved to maximize colonization. We now crete, symmetrical lichen thalli at locations age RAPD genetic divergence9 observed in show that sexually reproducing lichen- more than 10 m apart in south Wales, and five isolates of G. scripta from one wood- forming fungi can self-fertilize, and propose induced excised individual ascomata to land was 15.2% (according to a neighbour- that this breeding system allows these sym- discharge spores. Extraction of DNA from joining analysis of Jaccard’s coefficient of biotic organisms to reproduce successfully cultured mycelia derived from single spores band matching). Furthermore, ascospore in harsh environments. gave 218–263 randomly amplified poly- progeny from different ascomata on ‘single’ Most lichenized fungi produce abundant morphic DNA (RAPD) markers4 for each thalli of O. -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6. -

Evolution of Sexual Reproduction: a View from the Fungal Kingdom Supports an Evolutionary Epoch with Sex Before Sexes
fungal biology reviews xxx (2015) 1e10 journal homepage: www.elsevier.com/locate/fbr Review Evolution of sexual reproduction: A view from the fungal kingdom supports an evolutionary epoch with sex before sexes Joseph HEITMAN* Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, NC 27710, USA article info abstract Article history: Sexual reproduction is conserved throughout each supergroup within the eukaryotic tree Received 16 February 2015 of life, and therefore thought to have evolved once and to have been present in the last eu- Received in revised form karyotic common ancestor (LECA). Given the antiquity of sex, there are features of sexual 10 June 2015 reproduction that are ancient and ancestral, and thus shared in diverse extant organisms. Accepted 17 August 2015 On the other hand, the vast evolutionary distance that separates any given extant species from the LECA necessarily implies that other features of sex will be derived. While most Keywords: types of sex we are familiar with involve two opposite sexes or mating types, recent studies Evolution in the fungal kingdom have revealed novel and unusual patterns of sexual reproduction, Heterothallic including unisexual reproduction. In this mode of reproduction a single mating type can Homothallic on its own undergo self-fertile/homothallic reproduction, either with itself or with other Inbreeding members of the population of the same mating type. Unisexual reproduction has arisen MAT independently as a derived feature in several different lineages. That a myriad of different Mating type types of sex determination and sex determinants abound in animals, plants, protists, and Meiosis fungi suggests that sex specification itself may not be ancestral and instead may be a Outcrossing derived trait. -

Multiple Pathways to Homothallism in Closely Related Yeast Lineages in the Basidiomycota 2 3 Alexandra Cabrita1, Márcia David-Palma1*, Patrícia H
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.30.320192; this version posted December 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Multiple pathways to homothallism in closely related yeast lineages in the Basidiomycota 2 3 Alexandra Cabrita1, Márcia David-Palma1*, Patrícia H. Brito1, Joseph Heitman2, Marco A. Coelho1* and 4 Paula Gonçalves1# 5 # Corresponding author: [email protected] 6 1. Applied Molecular Biosciences Unit-UCIBIO, Departamento de Ciências da Vida, Faculdade de 7 Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal 8 2. Department of Molecular Genetics and Microbiology, Duke University, Duke University Medical 9 Center, Durham, NC 27710, USA. 10 Running title: Homothallism in Cystofilobasidium 11 12 Abstract 13 Sexual reproduction in fungi relies on proteins with well-known functions encoded by the mating-type 14 (MAT) loci. In the Basidiomycota, MAT loci are often bipartite, with the P/R locus encoding pheromone 15 precursors and pheromone receptors and the HD locus encoding heterodimerizing homeodomain 16 transcription factors (Hd1/Hd2). The interplay between different alleles of these genes within a single 17 species usually generates at least two compatible mating types. However, a minority of species are 18 homothallic, reproducing sexually without an obligate need for a compatible partner. Here we examine 19 the organization and function of the MAT loci of Cystofilobasidium capitatum, a species in the order 20 Cystofilobasidiales, which is unusually rich in homothallic species. -

Perspectives
Copyright 2000 by the Genetics Society of America Perspectives Anecdotal, Historical and Critical Commentaries on Genetics Edited by James F. Crow and William F. Dove Guido Pontecorvo (ªPonteº), 1907±1999 Bernard L. Cohen IBLS Division of Molecular Genetics, University of Glasgow, Glasgow G11 6NU, Scotland UIDO Pontecorvo died on September 25, 1999, and enjoy Aristophanes in Greek. During studies in the G age 91, of complications following a fall while col- Faculty of Agriculture in the University of Pisa, his inter- lecting mushrooms in his beloved Swiss mountains; he est in genetics was aroused by E. Avanzi, a plant geneti- was a signi®cant contributor to modern genetics. He cist. And in my recollection of a distant conversation, his was also an irascible yet genial friend and advisor who orientation toward agriculture resulted from working a attracted the great affection and admiration of col- relative's chicken farm when he was a teenager. Under- leagues and students worldwide and who, as head of graduate friends, among them Enrico Fermi (known department and Professor of Genetics, served the Uni- even then as ªthe Popeº because of his infallibility), were versity of Glasgow with distinction from 1945 until 1968. also in¯uential mountaineering and skiing companions. It was characteristic that in 1955, when promoted to the After two years' compulsory military service in the light newly created Chair of Genetics and also elected Fellow horse artilleryÐand somehow the image of Lieutenant of the Royal Society, he circulated a note saying that Pontecorvo exercising the commanding of®cer's horse henceforth the head of department should be known seems not at all incongruous!ÐPonte became assistant as ªPonteºÐmeaning of course no change; he was any- to Avanzi, now director of an experimental agricultural thing but pompous. -

Genomic Evidence for Independent Loss of the Canonical Synaptonemal Complex Sarah Shah1,2,3, Yibi Chen1,2,3, Debashish Bhattacharya4 & Cheong Xin Chan1,2,3 ✉
www.nature.com/scientificreports OPEN Sex in Symbiodiniaceae dinofagellates: genomic evidence for independent loss of the canonical synaptonemal complex Sarah Shah1,2,3, Yibi Chen1,2,3, Debashish Bhattacharya4 & Cheong Xin Chan1,2,3 ✉ Dinofagellates of the Symbiodiniaceae family encompass diverse symbionts that are critical to corals and other species living in coral reefs. It is well known that sexual reproduction enhances adaptive evolution in changing environments. Although genes related to meiotic functions were reported in Symbiodiniaceae, cytological evidence of meiosis and fertilisation are however yet to be observed in these taxa. Using transcriptome and genome data from 21 Symbiodiniaceae isolates, we studied genes that encode proteins associated with distinct stages of meiosis and syngamy. We report the absence of genes that encode main components of the synaptonemal complex (SC), a protein structure that mediates homologous chromosomal pairing and class I crossovers. This result suggests an independent loss of canonical SCs in the alveolates, that also includes the SC-lacking ciliates. We hypothesise that this loss was due in part to permanently condensed chromosomes and repeat-rich sequences in Symbiodiniaceae (and other dinofagellates) which favoured the SC-independent class II crossover pathway. Our results reveal novel insights into evolution of the meiotic molecular machinery in the ecologically important Symbiodiniaceae and in other eukaryotes. Sex is part of the life cycle of nearly all eukaryotes and has most likely been so since the last eukaryotic com- mon ancestor1. Even lineages that were traditionally thought to be asexual, such as the Amoebozoa, possess the molecular machinery required for sex2. Dinofagellates, a group of fagellated, mostly marine phytoplankton, are no exception. -

Birds Do It, Bees Do It, but Candida Albicans Does It Differently
Birds Do It, Bees Do It, but Candida albicans Does It Differently Richard Robinson | doi:10.1371/journal.pbio.0060121 The yeast Candida albicans lives an suggesting that many chromosomes do unnoticed and mostly harmless life as not harbor lethal recessive alleles, as a member of our gut flora. However, has been proposed for C. albicans. mainly in an immunocompromised Unexpectedly, the authors also host, it can proliferate and cause found evidence that, in some severe, life-threatening infections. strains, the remaining chromosomes Within this normally mild-mannered, had undergone homologous single-celled fungus beats the heart of recombination, one of the hallmarks a reproductive adventurer. For while of meiosis. C. albicans is known to it appears to be incapable of meiosis have genes that, in other organisms, and therefore true sex, it engages in an function specifically in meiosis, but no unusual and offbeat alternative—after role for them in C. albicans has been doi:10.1371/journal.pbio.0060121.g001 it mates, its progeny randomly cast off previously identified. The authors chromosomes to restore the diploid Configuration of the eight chromosomes showed that one such protein, Spo11p, number, or something close to it. In of Candida albicans in a recombinant strain which in other species cleaves double- a new study, Anja Forche, Richard derived from the parasexual mating cycle. stranded DNA as a prelude to meiotic Bennett, and colleagues show that this recombination, was expressed in process generates significant genetic been discovered, and its details and mitotically growing C. albicans. When diversity, which is further amplified by genetic consequences have not been the researchers deleted the gene, recombination between homologous well characterized. -

Sex, Outcrossing and Mating Types: Unsolved Questions in Fungi and Beyond
doi: 10.1111/j.1420-9101.2012.02495.x REVIEW Sex, outcrossing and mating types: unsolved questions in fungi and beyond S. BILLIARD*, M. LO´ PEZ-VILLAVICENCIO ,M.E.HOODà &T.GIRAUD§– *Laboratoire de Ge´ne´tique et Evolution des Populations Ve´ge´tales, UMR CNRS 8016, Universite´ des Sciences et Technologies de Lille – Lille1, Villeneuve d’Ascq Cedex, France Origine, Structure, Evolution de la Biodiversite´, UMR 7205 CNRS-MNHN, Muse´um National d’Histoire Naturelle, Paris Cedex, France àDepartment of Biology, McGuire Life Sciences Building, Amherst College, Amherst, MA, USA §Ecologie, Syste´matique et Evolution, Universite´ Paris-Sud, Orsay cedex, France –Ecologie, Syste´matique et Evolution, CNRS, Orsay cedex, France Keywords: Abstract ascomycete; Variability in the way organisms reproduce raises numerous, and still asexual reproduction; unsolved, questions in evolutionary biology. In this study, we emphasize that basidiomycete; fungi deserve a much greater emphasis in efforts to address these questions breeding systems; because of their multiple advantages as model eukaryotes. A tremendous diploid selfing; diversity of reproductive modes and mating systems can be found in fungi, gametophytic selfing; with many evolutionary transitions among closely related species. In addition, haploid selfing; fungi show some peculiarities in their mating systems that have received little mating systems; attention so far, despite the potential for providing insights into important oomycetes; evolutionary questions. In particular, selfing can occur at the haploid stage in sexual reproduction. addition to the diploid stage in many fungi, which is generally not possible in animals and plants but has a dramatic influence upon the structure of genetic systems. Fungi also present several advantages that make them tractable models for studies in experimental evolution.