Detailed Reconstruction of the Nervous and Muscular System Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylum Nemertea)
THE BIOLOGY AND SYSTEMATICS OF A NEW SPECIES OF RIBBON WORM, GENUS TUBULANUS (PHYLUM NEMERTEA) By Rebecca Kirk Ritger Submitted to the Faculty of the College of Arts and Sciences of American University in Partial Fulfillment of the Requirements for the Degree of Master of Science In Biology Chair: Dr. Qiristopher'Tudge m Dr.David C r. Jon L. Norenburg Dean of the College of Arts and Sciences JuK4£ __________ Date 2004 American University Washington, D.C. 20016 AMERICAN UNIVERSITY LIBRARY 1 1 0 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 1421360 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. ® UMI UMI Microform 1421360 Copyright 2004 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, Ml 48106-1346 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. THE BIOLOGY AND SYSTEMATICS OF A NEW SPECIES OF RIBBON WORM, GENUS TUBULANUS (PHYLUM NEMERTEA) By Rebecca Kirk Ritger ABSTRACT Most nemerteans are studied from poorly preserved museum specimens. -

A Phylum-Wide Survey Reveals Multiple Independent Gains of Head Regeneration Ability in Nemertea
bioRxiv preprint doi: https://doi.org/10.1101/439497; this version posted October 11, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. A phylum-wide survey reveals multiple independent gains of head regeneration ability in Nemertea Eduardo E. Zattara1,2,5, Fernando A. Fernández-Álvarez3, Terra C. Hiebert4, Alexandra E. Bely2 and Jon L. Norenburg1 1 Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA 2 Department of Biology, University of Maryland, College Park, MD, USA 3 Institut de Ciències del Mar, Consejo Superior de Investigaciones Científicas, Barcelona, Spain 4 Institute of Ecology and Evolution, University of Oregon, Eugene, OR, USA 5 INIBIOMA, Consejo Nacional de Investigaciones Científicas y Tecnológicas, Bariloche, RN, Argentina Corresponding author: E.E. Zattara, [email protected] Abstract Animals vary widely in their ability to regenerate, suggesting that regenerative abilities have a rich evolutionary history. However, our understanding of this history remains limited because regeneration ability has only been evaluated in a tiny fraction of species. Available comparative regeneration studies have identified losses of regenerative ability, yet clear documentation of gains is lacking. We surveyed regenerative ability in 34 species spanning the phylum Nemertea, assessing the ability to regenerate heads and tails either through our own experiments or from literature reports. Our sampling included representatives of the 10 most diverse families and all three orders comprising this phylum. -

Tubulanus Polymorphus Class: Anopla Order: Paleonemertea an Orange Ribbon Worm Family: Tubulanidae
Phylum: Nemertea Tubulanus polymorphus Class: Anopla Order: Paleonemertea An orange ribbon worm Family: Tubulanidae “Such a worm when seen crawling in long and Palaeonemertea) but with lateral graceful curves over the bottom in clear water transverse grooves (Fig. 2a, b, c). earns for itself a place among the most Head cannot completely withdraw into body (Kozloff 1974). beautiful of all marine invertebrates” (Coe Posterior: No caudal cirrus. 1905) Eyes/Eyespots: None. Mouth: A long slit-like opening (Fig. 2c) Taxonomy: Tubulanus polymorphous was a posterior to the brain, separate from name assigned in unpublished work by proboscis pore (Fig. 2c) and positioned just Renier (1804). The genera Tubulanus and behind transverse furrows (Coe 1901). Carinella were described by Renier (1804) Proboscis: Eversible (phylum Nemertea) and Johnston (1833), respectively, and were and, when not everted, coiled inside synonymized by Bürger in 1904 (Gibson rhynchocoel (cavity). The proboscis in 1995). Melville (1986) and the International Tubulanus polymorphus is short with the Code of Zoological Nomenclature (ICZN) rhynchocoel reaching one third total worm determined that the family name Tubulanidae body length. Proboscis bears no stylets and take precedence over its senior subjective the proboscis pore almost terminal (Fig. 2c). synonym Carinellidae (Ritger and Norenburg Tube/Burrow: As is true for most Tubulanus 2006) and the name Tubulanus polymorphus species, T. polymorphus individuals live in was deemed published and available (ICZN thin parchment tubes that are attached to 1988). Previous names for T. polymorphus rocks or shells and made of hardened include C. polymorpha, C. rubra and C. mucous secretions (Coe 1943). speciosa. Possible Misidentifications Description The genus Tubulanus is slender, soft, Size: A large nemertean, up to three meters extensible without ocelli or cephalic grooves when extended. -

Soil-Dwelling Polychaetes: Enigmatic As Ever? Some Hints on Their
Contributions to Zoology, 70 (3) 127-138 (2001) SPB Academic Publishing bv, The Hague Soil-dwelling polychaetes: enigmatic as ever? Some hints on their phylogenetic relationships as suggested by a maximum parsimony analysis of 18S rRNA gene sequences ³ Emilia Rota Patrick Martin² & Christer Erséus ¹, 1 di Dipartimento Biologia Evolutivei. Universitd di Siena, via P. A. Mattioli 4. IT-53100 Siena, Italy, e-mail: 2 Institut des Sciences naturelles de des [email protected]; royal Belgique, Biologic Eaux donees, 29 rue Vautier, B-1000 e-mail: 3 Bruxelles, Belgium, [email protected]; Department of Invertebrate Zoology, Swedish Museum of Natural History, Box 50007, SE-104 05 Stockholm, Sweden, e-mail: [email protected] Keywords: Terrestrial Polychaeta, Parergodrilus heideri, Stygocapitella subterranea, Hrabeiella I8S rRNA periglandulata, gene, molecular phylogeny, rapid radiation Abstract Collectionof new specimens 130 DNA extraction, amplification and sequencing 130 Alignment To re-evaluate 130 the various hypotheses on the systematic position of Phylogenetic analyses 130 Parergodrilus heideri Reisinger, 1925 and Hrabeiella Results 132 periglandulata Pizl & Chalupský, 1984,the sole truly terrestrial Discussion 132 non-clitellateannelidsknown to date, their phylogenetic relation- ships Acknowledgements 136 were investigated using a data set of new 18S rDNA References 136 of sequences these and other five relevant annelid taxa, including an unknown of species Ctenodrilidae, as well as homologous sequences available for 18 already polychaetes, one aphano- neuran, 11 clitellates, two pogonophorans, one echiuran, one Introduction sipunculan, three molluscs and two arthropods. Two different alignments were constructed, according to analgorithmic method terrestrial forms constitute (Clustal Truly a tiny minority W) and on the basis of a secondary structure model non-clitellate annelids, (DCSE), A maximum parsimony analysis was performed with among only represented by arthropods asan unambiguous outgroup. -

Resolving a 200-Year-Old Taxonomic Conundrum: Neotype Designation for Cephalothrix Linearis (Nemertea: Palaeonemertea) Based on a Topotype from Bergen, Norway
Fauna norvegica 2019 Vol. 39: 39–76. Resolving a 200-year-old taxonomic conundrum: neotype designation for Cephalothrix linearis (Nemertea: Palaeonemertea) based on a topotype from Bergen, Norway Hiroshi Kajihara1 Kajihara H. 2019. Resolving a 200-year-old taxonomic conundrum: neotype designation for Cephalothrix linearis (Nemertea: Palaeonemertea) based on a topotype from Bergen, Norway. Fauna norvegica 39: 39–76. The taxonomic identity of the palaeonemertean Cephalothrix linearis (Rathke, 1799) has been obscure for nearly two centuries, because its original description applies to almost any congeners, including Cephalothrix filiformis (Johnston 1828) and Cephalothrix rufifrons (Johnston, 1837), which occur commonly in the North Sea and adjacent waters. In this paper, I redescribe C. linearis based on two topotypes from Bergen, one herein designated as the neotype for C. linearis, because Rathke’s original material is not extant; I invoke Article 70.3.2 of the International Code of Zoological Nomenclature to fix Planaria linearis Rathke, 1799 as the type species of Cephalothrix Örsted, 1843 for the sake of stability. From the neotype, I determined sequences of the 28S rRNA, 16S rRNA, and cytochrome c oxidase subunit I (COI) genes. Using the COI sequence, I inferred the phylogenetic position of C. linearis along with 316 cephalotrichid sequences currently available in public databases. A tree-based species delimitation analysis detected 43 entities among them, with 34 in Cephalothrix and nine in either Balionemertes or Cephalotrichella. -

2020 Interim Receiving Waters Monitoring Report
POINT LOMA OCEAN OUTFALL MONTHLY RECEIVING WATERS INTERIM RECEIVING WATERS MONITORING REPORT FOR THE POINTM ONITORINGLOMA AND SOUTH R EPORTBAY OCEAN OUTFALLS POINT LOMA 2020 WASTEWATER TREATMENT PLANT NPDES Permit No. CA0107409 SDRWQCB Order No. R9-2017-0007 APRIL 2021 Environmental Monitoring and Technical Services 2392 Kincaid Road x Mail Station 45A x San Diego, CA 92101 Tel (619) 758-2300 Fax (619) 758-2309 INTERIM RECEIVING WATERS MONITORING REPORT FOR THE POINT LOMA AND SOUTH BAY OCEAN OUTFALLS 2020 POINT LOMA WASTEWATER TREATMENT PLANT (ORDER NO. R9-2017-0007; NPDES NO. CA0107409) SOUTH BAY WATER RECLAMATION PLANT (ORDER NO. R9-2013-0006 AS AMENDED; NPDES NO. CA0109045) SOUTH BAY INTERNATIONAL WASTEWATER TREATMENT PLANT (ORDER NO. R9-2014-0009 AS AMENDED; NPDES NO. CA0108928) Prepared by: City of San Diego Ocean Monitoring Program Environmental Monitoring & Technical Services Division Ryan Kempster, Editor Ami Latker, Editor June 2021 Table of Contents Production Credits and Acknowledgements ...........................................................................ii Executive Summary ...................................................................................................................1 A. Latker, R. Kempster Chapter 1. General Introduction ............................................................................................3 A. Latker, R. Kempster Chapter 2. Water Quality .......................................................................................................15 S. Jaeger, A. Webb, R. Kempster, -

On the Ground Pattern of Annelida**
Org. Divers. Evol. 2, 181–196 (2002) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ode On the ground pattern of Annelida** Günter Purschke* Spezielle Zoologie, Universität Osnabrück, Germany Received 7 March 2002 · Accepted 14 June 2002 Abstract Annelida, traditionally divided into Polychaeta and Clitellata, are characterized by serial division of their body into numerous similar structures, the segments. In addition, there is a non-segmental part at the front end, the prostomium, and one at the back, the pygidium. New segments develop in a prepygidial proliferation zone. Each segment contains four groups of chaetae made up of β-chitin, a pair of coelomic cavities sep- arated by mesenteries, and septa.The nervous system is a rope-ladder-like ventral nerve cord with a dorsal brain in the prostomium. For the last stem species a trochophore larva and a benthic adult are commonly postulated.There are two conflicting hypotheses describing the systemati- zation of Annelida: the first postulates a sister-group relationship of Polychaeta and Clitellata, the second sees Clitellata as a highly derived taxon forming a subordinate taxon within the polychaetes which, consequently, are regarded as paraphyletic. Depending on the hypothesis, different characters have to be postulated for the stem species of Annelida. Besides segmentation other characters such as nuchal organs, palps and antennae, body wall musculature, cuticle, parapodia as well as structure of the central nervous system and the foregut play an important role in this discussion. Here, the different characters and character states are critically reviewed and analyzed with respect to morphology and function.The consequences for systematization of their phylogenetic interpretation as autapomorphies, synapomorphies or plesiomorphies are outlined.The resulting hypotheses are compared with those relying on molecular data sets. -

A Taxonomic Catalogue of Japanese Nemerteans (Phylum Nemertea)
Title A Taxonomic Catalogue of Japanese Nemerteans (Phylum Nemertea) Author(s) Kajihara, Hiroshi Zoological Science, 24(4), 287-326 Citation https://doi.org/10.2108/zsj.24.287 Issue Date 2007-04 Doc URL http://hdl.handle.net/2115/39621 Rights (c) Zoological Society of Japan / 本文献の公開は著者の意思に基づくものである Type article Note REVIEW File Information zsj24p287.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP ZOOLOGICAL SCIENCE 24: 287–326 (2007) © 2007 Zoological Society of Japan [REVIEW] A Taxonomic Catalogue of Japanese Nemerteans (Phylum Nemertea) Hiroshi Kajihara* Department of Natural History Sciences, Faculty of Science, Hokkaido University, Sapporo 060-0810, Japan A literature-based taxonomic catalogue of the nemertean species (Phylum Nemertea) reported from Japanese waters is provided, listing 19 families, 45 genera, and 120 species as valid. Applications of the following species names to forms previously recorded from Japanese waters are regarded as uncertain: Amphiporus cervicalis, Amphiporus depressus, Amphiporus lactifloreus, Cephalothrix filiformis, Cephalothrix linearis, Cerebratulus fuscus, Lineus vegetus, Lineus bilineatus, Lineus gesserensis, Lineus grubei, Lineus longifissus, Lineus mcintoshii, Nipponnemertes pulchra, Oerstedia venusta, Prostoma graecense, and Prostoma grande. The identities of the taxa referred to by the fol- lowing four nominal species require clarification through future investigations: Cosmocephala japonica, Dicelis rubra, Dichilus obscurus, and Nareda serpentina. The nominal species established from Japanese waters are tabulated. In addition, a brief history of taxonomic research on Japanese nemerteans is reviewed. Key words: checklist, Pacific, classification, ribbon worm, Nemertinea 2001). The only recent listing of previously described Japa- INTRODUCTION nese species is the checklist of Crandall et al. (2002), but The phylum Nemertea comprises about 1,200 species the relevant literature is scattered. -

Fauna Europaea: Annelida - Terrestrial Oligochaeta (Enchytraeidae and Megadrili), Aphanoneura and Polychaeta
Biodiversity Data Journal 3: e5737 doi: 10.3897/BDJ.3.e5737 Data Paper Fauna Europaea: Annelida - Terrestrial Oligochaeta (Enchytraeidae and Megadrili), Aphanoneura and Polychaeta Emilia Rota‡, Yde de Jong §,| ‡ University of Siena, Siena, Italy § University of Amsterdam - Faculty of Science, Amsterdam, Netherlands | Museum für Naturkunde, Berlin, Germany Corresponding author: Emilia Rota ([email protected]), Yde de Jong ([email protected]) Academic editor: Christos Arvanitidis Received: 26 Jul 2015 | Accepted: 07 Sep 2015 | Published: 11 Sep 2015 Citation: Rota E, de Jong Y (2015) Fauna Europaea: Annelida - Terrestrial Oligochaeta (Enchytraeidae and Megadrili), Aphanoneura and Polychaeta. Biodiversity Data Journal 3: e5737. doi: 10.3897/BDJ.3.e5737 Abstract Fauna Europaea provides a public web-service with an index of scientific names (including important synonyms) of all living European land and freshwater animals, their geographical distribution at country level (up to the Urals, excluding the Caucasus region), and some additional information. The Fauna Europaea project covers about 230,000 taxonomic names, including 130,000 accepted species and 14,000 accepted subspecies, which is much more than the originally projected number of 100,000 species. This represents a huge effort by more than 400 contributing specialists throughout Europe and is a unique (standard) reference suitable for many users in science, government, industry, nature conservation and education. This paper provides updated information on the taxonomic composition and distribution of the Annelida - terrestrial Oligochaeta (Megadrili and Enchytraeidae), Aphanoneura and Polychaeta, recorded in Europe. Data on 18 families, 11 autochthonous and 7 allochthonous, represented in our continent by a total of 800 species, are reviewed, beginning from their distinctness, phylogenetic status, diversity and global distribution, and following with major recent developments in taxonomic and faunistic research in Europe. -

Tetrodotoxin and Its Analogues in Cephalothrix Cf. Simula
toxins Article Tetrodotoxin and Its Analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody Distribution and Secretions Anna E. Vlasenko and Timur Yu. Magarlamov * A.V. Zhirmunsky National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Sciences, 690041 Vladivostok, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-914-661-7949 Received: 15 November 2020; Accepted: 25 November 2020; Published: 26 November 2020 Abstract: Some nemertean species from the genus Cephalothrix accumulate tetrodotoxin (TTX) in extremely high concentrations. The current study is the first to provide high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) data on tetrodotoxin and its analogues (TTXs) profile and concentration in different regions and organs of Cephalothrix cf. simula, and its secretions produced in response to stimulation. Different specimens of C. cf. simula possessed 7–11 analogues, including nine previously found in this species and two new for nemerteans—4,9-anhydro-8-epi-5,6,11-trideoxyTTX and 1-hydroxy-8-epi-5,6,11-trideoxyTTX. The study of the toxins’ distribution in different regions and organs of nemerteans revealed the same qualitative composition of TTXs throughout the body but differences in the total concentration of the toxins. The total concentration of TTXs was highest in the anterior region of the body and decreased towards the posterior; the ratio of the analogues also differed between regions. The data obtained suggest a pathway of TTXs uptake in C. cf. simula and the role of toxins in the life activity of nemerteans. -
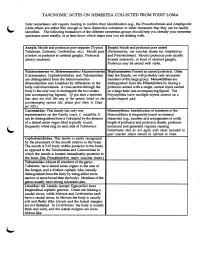
Taxonomic Notes on Nemertea Collected from Point Loma
TAXONOMIC NOTES ON NEMERTEA COLLECTED FROM POINT LOMA Some nemerteans will require clearing to confirm their identification (e.g., the Prosorhochmids and Amphiporid: while others are either thin enough or have distinctive coloration or other characters that they can be readily identified. The following breakdown of the different nemertean groups should help you identify your nemertear specimens more readily, or at least know which major taxa you are dealing with. Anopla: Mouth and proboscis pore separate (Typical Enopla: Mouth and proboscis pore united Tubulanus, Carinoma, Cerebratulus, etc.). Mouth pore (Paranemertes, see voucher sheets for Amphiporus is below or posterior to cerebral ganglia. Proboscis and Prosorhochmus). Mouth/proboscis pore usually always unarmed. located anteriorly, in front of cerebral ganglia. Proboscis may be armed with stylet. Palaeonemertea vs. Heteronemertea: Palaeonemertea Hoplonemertea: Possess an armed proboscis. Other (Carinomidae, Cephalotrichidae, and Tubulanidae) than the Enopla, we will probably only encounter are distinguished from the Heteronemertea members of this large group. Monostylif era are (Baseodiscidae and Lineidae) by differences in the distinguished from the Polystylifera by having a body wall musculature. A cross section through the proboscis armed with a single, central stylet carried body is the only way to distinguish the two orders on a large basis (see accompanying figure). The (see accompanying figures). (If you have a specimen Polystylifera have multiple stylets carried on a that does not look like any of the species listed on the sickle-shaped pad. accompanying species list, please give them to Dean for FID.) Carinomidae: This family has only one Monostylifera: Identification of members of the representative on the Pacific coast, C. -

Zootaxa 1668:245–264 (2007) ISSN 1175-5326 (Print Edition) ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (Online Edition)
Zootaxa 1668:245–264 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Annelida* GREG W. ROUSE1 & FREDRIK PLEIJEL2 1Scripps Institution of Oceanography, UCSD, 9500 Gilman Drive, La Jolla CA, 92093-0202, USA. E-mail: [email protected] 2Department of Marine Ecology, Tjärnö Marine Biological Laboratory, Göteborg University, SE-452 96 Strömstad, Sweden. E-mail: [email protected] *In: Zhang, Z.-Q. & Shear, W.A. (Eds) (2007) Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa, 1668, 1–766. Table of contents Abstract . .245 Introduction . .245 Major polychaete taxa . .250 Monophyly of Annelida . .255 Molecular sequence data . 258 Rooting the annelid tree . .259 References . 261 Abstract The first annelids were formally described by Linnaeus (1758) and we here briefly review the history and composition of the group. The traditionally recognized classes were Polychaeta, Oligochaeta and Hirudinea. The latter two are now viewed as the taxon Clitellata, since recognizing Hirudinea with class rank renders Oligochaeta paraphyletic. Polychaeta appears to contain Clitellata, and so may be synonymous with Annelida. Current consensus would place previously rec- ognized phyla such as Echiura, Pogonophora, Sipuncula and Vestimentifera as annelids, though relationships among these and the various other annelid lineages are still unresolved. Key words: Polychaeta, Oligochaeta, Clitellata, Echiura, Pogonophora, Vestimentifera, Sipuncula, phylogeny, review Introduction Annelida is a group commonly referred to as segmented worms, found worldwide in terrestrial, freshwater and marine habitats. The first annelids were formally named by Linnaeus, including well-known forms such as the earthworm Lumbricus terrestris Linnaeus, 1758, the medicinal leech Hirudo medicinalis Linnaeus, 1758, and the sea-mouse Aphrodite aculeata Linnaeus, 1758.