Fine Structure of Rat Intrafusal Muscle Fibers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1933.Full.Pdf
0270.6474/84/0408-1933$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 8, pp. 1933-1943 Printed in U.S.A. August 1984 PARTIAL PURIFICATION AND FUNCTIONAL IDENTIFICATION OF A CALMODULIN-ACTIVATED, ADENOSINE 5’-TRIPHOSPHATE-DEPENDENT CALCIUM PUMP FROM SYNAPTIC PLASMA MEMBRANES1 DIANE M. PAPAZIAN,* HANNAH RAHAMIMOFF,$ AND STANLEY M. GOLDIN§’ Departments of *Biological Chemistry and $Pharmacology, Harvard Medical School, Boston, Massachusetts 02115 and *Department of Biochemistry, Hadassah Medical School, Hebrew University, Jerusalem, Israel Received July 18, 1983; Revised February 27, 1984; Accepted February 28, 1984 Abstract Synaptic plasma membranes isolated from rat brain contain a calmodulin-activated Ca2+ pump. It has been purified 80- to 160-fold by solubilization with Triton X-100 and affinity chromatography on a calmodulin- Sepharose 4B column. After reconstitution into phospholipid vesicles, the affinity-purified pump efficiently catalyzed ATP dependent Ca2+ transport, which was activated 7- to g-fold by calmodulin. The major protein component of the affinity-purified preparation had a M, = 140,000; it was virtually the only band visualized on a Coomassie blue-stained SDS polyacrylamide gel. It has been identified as the Ca”’ pump by two functional criteria. First, it was phosphorylated by [y-““P]ATP in a Ca’+-dependent manner; the phosphorylated protein had the chemical reactivity of an acyl phosphate, characteristic of the phosphorylated intermediates of ion-transporting ATPases. Second, the protein was enriched by transport-specific fractiona- tion, a density gradient procedure which uses the transport properties of the reconstituted Ca2+ pump as a physical tool for its purification. -

Microanatomy of Muscles
Microanatomy of Muscles Anatomy & Physiology Class Three Main Muscle Types Objectives: By the end of this presentation you will have the information to: 1. Describe the 3 main types of muscles. 2. Detail the functions of the muscle system. 3. Correctly label the parts of a myocyte (muscle cell) 4. Identify the levels of organization in a skeletal muscle from organ to myosin. 5. Explain how a muscle contracts utilizing the correct terminology of the sliding filament theory. 6. Contrast and compare cardiac and smooth muscle with skeletal muscle. Major Functions: Muscle System 1. Moving the skeletal system and posture. 2. Passing food through the digestive system & constriction of other internal organs. 3. Production of body heat. 4. Pumping the blood throughout the body. 5. Communication - writing and verbal Specialized Cells (Myocytes) ~ Contractile Cells Can shorten along one or more planes because of specialized cell membrane (sarcolemma) and specialized cytoskeleton. Specialized Structures found in Myocytes Sarcolemma: The cell membrane of a muscle cell Transverse tubule: a tubular invagination of the sarcolemma of skeletal or cardiac muscle fibers that surrounds myofibrils; involved in transmitting the action potential from the sarcolemma to the interior of the myofibril. Sarcoplasmic Reticulum: The special type of smooth endoplasmic Myofibrils: reticulum found in smooth and a contractile fibril of skeletal muscle, composed striated muscle fibers whose function mainly of actin and myosin is to store and release calcium ions. Multiple Nuclei (skeletal) & many mitochondria Skeletal Muscle - Microscopic Anatomy A whole skeletal muscle (such as the biceps brachii) is considered an organ of the muscular system. Each organ consists of skeletal muscle tissue, connective tissue, nerve tissue, and blood or vascular tissue. -

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

(7E) Powerpoint Lecture Outline Chapter 8: Control of Movement
Carlson (7e) PowerPoint Lecture Outline Chapter 8: Control of Movement This multimedia product and its contents are protected under copyright law. The following are prohibited by law: •any public performance or display, including transmission of any image over a network; •preparation of any derivative work, including extraction, in whole or in part, of any images; •any rental, lease, or lending of the program. Copyright 2001 by Allyn & Bacon Skeletal Muscle n Movements of our body are accomplished by contraction of the skeletal muscles l Flexion: contraction of a flexor muscle draws in a limb l Extension: contraction of extensor muscle n Skeletal muscle fibers have a striated appearance n Skeletal muscle is composed of two fiber types: l Extrafusal: innervated by alpha-motoneurons from the spinal cord: exert force l Intrafusal: sensory fibers that detect stretch of the muscle u Afferent fibers: report length of intrafusal: when stretched, the fibers stimulate the alpha-neuron that innervates the muscle fiber: maintains muscle tone u Efferent fibers: contraction adjusts sensitivity of afferent fibers. 8.2 Copyright 2001 by Allyn & Bacon Skeletal Muscle Anatomy n Each muscle fiber consists of a bundle of myofibrils l Each myofibril is made up of overlapping strands of actin and myosin l During a muscle twitch, the myosin filaments move relative to the actin filaments, thereby shortening the muscle fiber 8.3 Copyright 2001 by Allyn & Bacon Neuromuscular Junction n The neuromuscular junction is the synapse formed between an alpha motor neuron -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
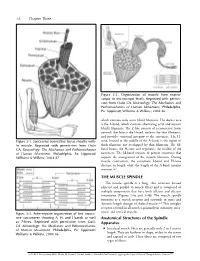
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

CAP2 Deficiency Delays Myofibril Actin Cytoskeleton Differentiation and Disturbs Skeletal Muscle Architecture and Function
CAP2 deficiency delays myofibril actin cytoskeleton differentiation and disturbs skeletal muscle architecture and function Lara-Jane Kepsera, Fidan Damara, Teresa De Ciccob, Christine Chaponnierc, Tomasz J. Prószynski b, Axel Pagenstecherd, and Marco B. Rusta,e,f,1 aMolecular Neurobiology Group, Institute of Physiological Chemistry, University of Marburg, 35032 Marburg, Germany; bLaboratory of Synaptogenesis, Nencki Institute of Experimental Biology PAS, 02-093 Warsaw, Poland; cDepartment of Pathology and Immunology, University of Geneva, 1211 Geneva, Switzerland; dInstitute of Neuropathology, University of Marburg, 35032 Marburg, Germany; eCenter for Mind, Brain and Behavior, Research Campus of Central Hessen, 35032 Marburg, Germany; and fDFG Research Training Group “Membrane Plasticity in Tissue Development and Remodeling,” GRK 2213, University of Marburg, 35032 Marburg, Germany Edited by Yale E. Goldman, University of Pennsylvania/PMI, Philadelphia, PA, and approved March 14, 2019 (received for review August 7, 2018) Actin filaments (F-actin) are key components of sarcomeres, the have acquired specific functions. While previous analyses of mu- basic contractile units of skeletal muscle myofibrils. A crucial step tant mice demonstrated a role of CAP2 in neuron morphology and during myofibril differentiation is the sequential exchange of heart physiology (13–15), its function in skeletal muscles has not α-actin isoforms from smooth muscle (α-SMA) and cardiac (α-CAA) been investigated, yet. to skeletal muscle α-actin (α-SKA) that, in mice, occurs during early We here report a function for CAP2 in skeletal muscle de- postnatal life. This “α-actin switch” requires the coordinated activ- velopment. We found that CAP2 controls the exchange of ity of actin regulators because it is vital that sarcomere structure α-actin isoforms during myofibril differentiation. -

Muscle Physiology Dr
Muscle Physiology Dr. Ebneshahidi Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Skeletal Muscle Figure 9.2 (a) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Functions of the muscular system . 1. Locomotion . 2. Vasoconstriction and vasodilatation- constriction and dilation of blood vessel Walls are the results of smooth muscle contraction. 3. Peristalsis – wavelike motion along the digestive tract is produced by the Smooth muscle. 4. Cardiac motion . 5. Posture maintenance- contraction of skeletal muscles maintains body posture and muscle tone. 6. Heat generation – about 75% of ATP energy used in muscle contraction is released as heat. Copyright. © 2004 Pearson Education, Inc., publishing as Benjamin Cummings . Striation: only present in skeletal and cardiac muscles. Absent in smooth muscle. Nucleus: smooth and cardiac muscles are uninculcated (one nucleus per cell), skeletal muscle is multinucleated (several nuclei per cell ). Transverse tubule ( T tubule ): well developed in skeletal and cardiac muscles to transport calcium. Absent in smooth muscle. Intercalated disk: specialized intercellular junction that only occurs in cardiac muscle. Control: skeletal muscle is always under voluntary control‚ with some exceptions ( the tongue and pili arrector muscles in the dermis). smooth and cardiac muscles are under involuntary control. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Innervation: motor unit . a) a motor nerve and a myofibril from a neuromuscular junction where gap (called synapse) occurs between the two structures. at the end of motor nerve‚ neurotransmitter (i.e. acetylcholine) is stored in synaptic vesicles which will release the neurotransmitter using exocytosis upon the stimulation of a nerve impulse. Across the synapse the surface the of myofibril contains receptors that can bind with the neurotransmitter. -

Massive Alterations of Sarcoplasmic Reticulum Free Calcium in Skeletal Muscle Fibers Lacking Calsequestrin Revealed by a Genetic
Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe M. Canatoa,1, M. Scorzetoa,1, M. Giacomellob, F. Protasic,d, C. Reggiania,d, and G. J. M. Stienene,2 Departments of aHuman Anatomy and Physiology and bExperimental Veterinary Sciences, University of Padua, 35121 Padua, Italy; cCeSI, Department of Basic and Applied Medical Sciences, University G. d’Annunzio, I-66013 Chieti, Italy; dIIM-Interuniversity Institute of Myology and eLaboratory for Physiology, Institute for Cardiovascular Research, VU University Medical Center, 1081BT, Amsterdam, The Netherlands Edited* by Clara Franzini-Armstrong, University of Pennsylvania Medical Center, Philadelphia, PA, and approved November 12, 2010 (received for review June 30, 2010) The cytosolic free Ca2+ transients elicited by muscle fiber excitation tion essential to maintain a low metabolic rate in quiescent ex- are well characterized, but little is known about the free [Ca2+] citable cells. In addition, CSQ has been shown to modulate RyR- dynamics within the sarcoplasmic reticulum (SR). A targetable mediated Ca2+ release from the SR (6). To understand the pivotal ratiometric FRET-based calcium indicator (D1ER Cameleon) allowed role of CSQ in SR function, it is critical to determine free SR us to investigate SR Ca2+ dynamics and analyze the impact of cal- Ca2+ concentration, and this has been made possible by the ad- sequestrin (CSQ) on SR [Ca2+] in enzymatically dissociated flexor vent of a targetable ratiometric FRET-based indicator, such as digitorum brevis muscle fibers from WT and CSQ-KO mice lacking D1ER Cameleon (7). Seminal studies using this technique have isoform 1 (CSQ-KO) or both isoforms [CSQ-double KO (DKO)]. -

Localization of Ca2l Release Channels with Ryanodine in Junctional
Proc. NatI. Acad. Sci. USA Vol. 82, pp. 7256-7259, November 1985 Biochemistry Localization of Ca2l release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle (muscle contraction/excitation-contraction coupling/ruthenium red/longitudinal cisternae/gated channels) SIDNEY FLEISCHER, EUNICE M. OGUNBUNMI, MARK C. DIXON, AND EDUARD A. M. FLEER Department of Molecular Biology, Vanderbilt University, Nashville, TN 37235 Communicated by William J. Darby, July 8, 198S ABSTRACT The mechanism of Ca2' release from tional terminal cisternae consist of two types of membranes, sarcoplasmic reticulum, which triggers contraction in skeletal the Ca2l pump membrane (80-85%) and the junctional face muscle, remains the key unresolved problem in excita- membrane (15-20%) (16). A unique characteristic of junc- tion-contraction coupling. Recently, we have described the tional terminal cisternae is that they have a poor Ca2+ loading isolation of purified fractions referable to terminal and longi- rate, which can be enhanced 5-fold or more by addition of tudinal cisternae of sarcoplasmic reticulum. Junctional termi- ruthenium red (RR) (ref. 17; unpublished data). This study nal cisternae are distinct in that they have a low net energized describes the drug action of ryanodine on the junctional Ca2+ loading, which can be enhanced 5-fold or more by terminal cisternae in blocking the action of RR. It provides addition of ruthenium red. The loading rate, normalized for evidence that the action of ryanodine is on the Ca2' release calcium pump protein content, then approaches that of longi- mechanism and supports the view that Ca2' release is tudinal cisternae of sarcoplasmic reticulum.