Titin N2A Domain and Its Interactions at the Sarcomere
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Wiskott-Aldrich Syndrome: the Actin Cytoskeleton and Immune Cell Function
Disease Markers 29 (2010) 157–175 157 DOI 10.3233/DMA-2010-0735 IOS Press The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function Michael P. Blundella, Austen Wortha,b, Gerben Boumaa and Adrian J. Thrashera,b,∗ aMolecular Immunology Unit, UCL Institute of Child Health, London, UK bDepartment of Immunology, Great Ormond Street Hospital NHS Trust, Great Ormond Street, London, UK Abstract. Wiskott-Aldrich syndrome (WAS) is a rare X-linked recessive primary immunodeficiency characterised by immune dysregulation, microthrombocytopaenia, eczema and lymphoid malignancies. Mutations in the WAS gene can lead to distinct syndrome variations which largely, although not exclusively, depend upon the mutation. Premature termination and deletions abrogate Wiskott-Aldrich syndrome protein (WASp) expression and lead to severe disease (WAS). Missense mutations usually result in reduced protein expression and the phenotypically milder X-linked thrombocytopenia (XLT) or attenuated WAS [1–3]. More recently however novel activating mutations have been described that give rise to X-linked neutropenia (XLN), a third syndrome defined by neutropenia with variable myelodysplasia [4–6]. WASP is key in transducing signals from the cell surface to the actin cytoskeleton, and a lack of WASp results in cytoskeletal defects that compromise multiple aspects of normal cellular activity including proliferation, phagocytosis, immune synapse formation, adhesion and directed migration. Keywords: Wiskott-Aldrich syndrome, actin polymerization, lymphocytes, -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

Appropriate Roles of Cardiac Troponins in Evaluating Patients with Chest Pain
J Am Board Fam Pract: first published as 10.3122/jabfm.12.3.214 on 1 May 1999. Downloaded from MEDICAL PRACTICE Appropriate Roles of Cardiac Troponins in Evaluating Patients With Chest Pain Matthew S. Rice, MD, CPT, Me, USA, and David C. MacDonald, DO, Me, USA Background: Diagnosis of acute myocardial infarction relies upon the clinical history, interpretation of the electrocardiogram, and measurement of serum levels of cardiac enzymes. Newer biochemical markers of myocardial injury, such as cardiac troponin I and cardiac troponin T, are now being used instead of or along with the standard markers, the MB isoenzyme of creatine kinase (CK-MB) and lactate dehydrogenase. Methods: We performed a MEDLINE literature search (1987 to 1997) using the key words "troponin I," "troponin T," and "acute myocardial infarction." We reviewed selected articles related to the diagnostic and prognostic usefulness of these cardiac markers in evaluating patients with suspected myocardial infarction. Results: We found that (1) troponin I is a better cardiac marker than CK-MB for myocardial infarction because it is equally sensitive yet more specific for myocardial injury; (2) troponin T is a relatively poorer cardiac marker than CK-MB because it is less sensitive and less specific for myocardial injury; and (3) both troponin I and troponin T may be used as independent prognosticators of future cardiac events. Conclusions: Troponin I is a sensitive and specific marker for myocardial injury and can be used to predict the likelihood of future cardiac events. It is not much more expensive to measure than CK-MB. Over all, troponin I is a better cardiac marker than CK-MB and should become the preferred cardiac enzyme when evaluating patients with suspected myocardial infarction. -

Development of a High-Throughput Screen to Identify Small Molecule Enhancers of Sarcospan for the Treatment of Duchenne Muscular Dystrophy
UCLA UCLA Previously Published Works Title Development of a high-throughput screen to identify small molecule enhancers of sarcospan for the treatment of Duchenne muscular dystrophy. Permalink https://escholarship.org/uc/item/85z6k8t7 Journal Skeletal muscle, 9(1) ISSN 2044-5040 Authors Shu, Cynthia Kaxon-Rupp, Ariana N Collado, Judd R et al. Publication Date 2019-12-12 DOI 10.1186/s13395-019-0218-x Peer reviewed eScholarship.org Powered by the California Digital Library University of California Shu et al. Skeletal Muscle (2019) 9:32 https://doi.org/10.1186/s13395-019-0218-x RESEARCH Open Access Development of a high-throughput screen to identify small molecule enhancers of sarcospan for the treatment of Duchenne muscular dystrophy Cynthia Shu1,2,3, Ariana N. Kaxon-Rupp2, Judd R. Collado2, Robert Damoiseaux4,5 and Rachelle H. Crosbie1,2,3,6* Abstract Background: Duchenne muscular dystrophy (DMD) is caused by loss of sarcolemma connection to the extracellular matrix. Transgenic overexpression of the transmembrane protein sarcospan (SSPN) in the DMD mdx mouse model significantly reduces disease pathology by restoring membrane adhesion. Identifying SSPN-based therapies has the potential to benefit patients with DMD and other forms of muscular dystrophies caused by deficits in muscle cell adhesion. Methods: Standard cloning methods were used to generate C2C12 myoblasts stably transfected with a fluorescence reporter for human SSPN promoter activity. Assay development and screening were performed in a core facility using liquid handlers and imaging systems specialized for use with a 384-well microplate format. Drug-treated cells were analyzed for target gene expression using quantitative PCR and target protein expression using immunoblotting. -

The Uvomorulin-Anchorage Protein a Catenin Is a Vinculin
Proc. Nail. Acad. Sci. USA Vol. 88, pp. 9156-9160, October 1991 Cell Biology The uvomorulin-anchorage protein a catenin is a vinculin homologue KURT HERRENKNECHT*, MASAYUKI OZAWA*t, CHRISTOPH ECKERSKORN*, FRIEDRICH LOTTSPEICHt, MARTIN LENTER*, AND ROLF KEMLER*§ *Max-Planck-Institut ffir Immunbiologie, FG Molekulare Embryologie, D-7800 Freiburg, Federal Republic of Germany; and tMax-Planck-Institut ffr Biochemie, D-8033 Martinsried, Federal Republic of Germany Communicated by Franqois Jacob, July 18, 1991 (receivedfor review June 25, 1991) ABSTRACT The cytoplasmic region of the Ca2+- domain is well conserved in other cadherins, it is possible that dependent cell-adhesion molecule (CAM) uvomorulin associ- catenins may also complex with other members of this gene ates with distinct cytoplasmic proteins with molecular masses family (13, 14). Here we have produced antibodies against a of 102, 88, and 80 kDa termed a, (3, and ycatenin, respectively. catenin and show that a catenin is indeed associated with This complex formation links uvomorulin to the actin filament cadherins from human, mouse, and Xenopus. We have network, which seems to be of primary importance for its cloned and sequenced¶ the cDNA coding for a catenin and cell-adhesion properties. We show here that antibodies against have established the primary protein structure. Sequence a catenin also immunoprecipitate complexes that contain hu- comparison reveals homology to vinculin, a well-known man N-cadherin, mouse P-cadherin, chicken A-CAM (adhe- adherens-type and focal contact protein. rens junction-specific CAM; also called N-cadherin) or Xeno- pus U-cadherin, demonstrating that a catenin is complexed with other cadherins. -

<Alpha>11<Beta>1 Integrin Is a Receptor for Interstitial Collagens
Developmental Biology 237, 116–129 (2001) doi:10.1006/dbio.2001.0363, available online at http://www.idealibrary.com on View metadata, citation and similar papers at core.ac.uk brought to you by CORE ␣111 Integrin Is a Receptor for Interstitial provided by Elsevier - Publisher Connector Collagens Involved in Cell Migration and Collagen Reorganization on Mesenchymal Nonmuscle Cells Carl-Fredrik Tiger,* Francoise Fougerousse,† Gunilla Grundstro¨m,‡ Teet Velling,‡ and Donald Gullberg‡,1 *Department of Cell and Molecular Biology, Biomedical Center, Box 596, Uppsala University, S-75124 Uppsala, Sweden; †Laboratoire de Histoembryologie et de Cytoge´ne´tique, Faculte´ Cochin Port Royal, Paris 75014, France; and ‡Department of Medical Biochemistry and Microbiology, Biomedical Center, Box 582, Uppsala University, S-75123 Uppsala, Sweden ␣111 integrin constitutes a recent addition to the integrin family. Here, we present the first in vivo analysis of ␣11 protein and mRNA distribution during human embryonic development. ␣11 protein and mRNA were present in various mesenchymal cells around the cartilage anlage in the developing skeleton in a pattern similar to that described for the transcription factor scleraxis. ␣11 was also expressed by mesenchymal cells in intervertebral discs and in keratocytes in cornea, two sites with highly organized collagen networks. Neither ␣11 mRNA nor ␣11 protein could be detected in myogenic cells in human embryos. The described expression pattern is compatible with ␣111 functioning as a receptor for interstitial collagens in vivo. To test this hypothesis in vitro, full-length human ␣11 cDNA was stably transfected into the mouse satellite cell line C2C12, lacking endogenous collagen receptors. -

Removal of Abnormal Myofilament O-Glcnacylation Restores Ca
Diabetes Volume 64, October 2015 3573 Genaro A. Ramirez-Correa,1 Junfeng Ma,2 Chad Slawson,3 Quira Zeidan,2 Nahyr S. Lugo-Fagundo,1 Mingguo Xu,1 Xiaoxu Shen,4 Wei Dong Gao,4 Viviane Caceres,5 Khalid Chakir,5 Lauren DeVine,2 Robert N. Cole,2 Luigi Marchionni,6 Nazareno Paolocci,5 Gerald W. Hart,2 and Anne M. Murphy1 Removal of Abnormal Myofilament O-GlcNAcylation Restores Ca2+ Sensitivity in Diabetic Cardiac Muscle Diabetes 2015;64:3573–3587 | DOI: 10.2337/db14-1107 Contractile dysfunction and increased deposition of In diabetic cardiomyopathy, the contractile and electro- O-linked b-N-acetyl-D-glucosamine (O-GlcNAc) in car- physiological properties of the cardiac muscle are altered diac proteins are a hallmark of the diabetic heart. How- (1). Prior studies have mainly focused on alterations in ever, whether and how this posttranslational alteration Ca2+ handling (2–4). However, these perturbations alone contributes to lower cardiac function remains unclear. unlikely account for lower force production and altered COMPLICATIONS fi b Using a re ned -elimination/Michael addition with tan- relaxation typically found in the heart of patients with dem mass tags (TMT)–labeling proteomic technique, we diabetes (5,6). Indeed, the intrinsic properties of cardiac show that CpOGA, a bacterial analog of O-GlcNAcase myofilaments appear to be altered too (7,8). More specif- (OGA) that cleaves O-GlcNAc in vivo, removes site- 2+ 2+ fi specific O-GlcNAcylation from myofilaments, restoring ically, Ca sensitivity (ECa 50), a measure of myo la- 2+ Ca2+ sensitivity in streptozotocin (STZ) diabetic cardiac ment force production at near physiological Ca levels, – muscles. -

Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D
American Journal of Gastroenterology ISSN 0002-9270 C 2006 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2006.00375.x Published by Blackwell Publishing CME Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D. Foulkes, M.B., Ph.D.2 1Division of Gastroenterology, Department of Medicine, The Sir Mortimer B. Davis Jewish General Hospital, McGill University, Montreal, Quebec, Canada, and 2Program in Cancer Genetics, Departments of Oncology and Human Genetics, McGill University, Montreal, Quebec, Canada Familial adenomatous polyposis (FAP) is an autosomal-dominant colorectal cancer syndrome, caused by a germline mutation in the adenomatous polyposis coli (APC) gene, on chromosome 5q21. It is characterized by hundreds of adenomatous colorectal polyps, with an almost inevitable progression to colorectal cancer at an average age of 35 to 40 yr. Associated features include upper gastrointestinal tract polyps, congenital hypertrophy of the retinal pigment epithelium, desmoid tumors, and other extracolonic malignancies. Gardner syndrome is more of a historical subdivision of FAP, characterized by osteomas, dental anomalies, epidermal cysts, and soft tissue tumors. Other specified variants include Turcot syndrome (associated with central nervous system malignancies) and hereditary desmoid disease. Several genotype–phenotype correlations have been observed. Attenuated FAP is a phenotypically distinct entity, presenting with fewer than 100 adenomas. Multiple colorectal adenomas can also be caused by mutations in the human MutY homologue (MYH) gene, in an autosomal recessive condition referred to as MYH associated polyposis (MAP). Endoscopic screening of FAP probands and relatives is advocated as early as the ages of 10–12 yr, with the objective of reducing the occurrence of colorectal cancer. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -

Profiling of the Muscle-Specific Dystroglycan Interactome Reveals the Role of Hippo Signaling in Muscular Dystrophy and Age-Dependent Muscle Atrophy Andriy S
Yatsenko et al. BMC Medicine (2020) 18:8 https://doi.org/10.1186/s12916-019-1478-3 RESEARCH ARTICLE Open Access Profiling of the muscle-specific dystroglycan interactome reveals the role of Hippo signaling in muscular dystrophy and age-dependent muscle atrophy Andriy S. Yatsenko1†, Mariya M. Kucherenko2,3,4†, Yuanbin Xie2,5†, Dina Aweida6, Henning Urlaub7,8, Renate J. Scheibe1, Shenhav Cohen6 and Halyna R. Shcherbata1,2* Abstract Background: Dystroglycanopathies are a group of inherited disorders characterized by vast clinical and genetic heterogeneity and caused by abnormal functioning of the ECM receptor dystroglycan (Dg). Remarkably, among many cases of diagnosed dystroglycanopathies, only a small fraction can be linked directly to mutations in Dg or its regulatory enzymes, implying the involvement of other, not-yet-characterized, Dg-regulating factors. To advance disease diagnostics and develop new treatment strategies, new approaches to find dystroglycanopathy-related factors should be considered. The Dg complex is highly evolutionarily conserved; therefore, model genetic organisms provide excellent systems to address this challenge. In particular, Drosophila is amenable to experiments not feasible in any other system, allowing original insights about the functional interactors of the Dg complex. Methods: To identify new players contributing to dystroglycanopathies, we used Drosophila as a genetic muscular dystrophy model. Using mass spectrometry, we searched for muscle-specific Dg interactors. Next, in silico analyses allowed us to determine their association with diseases and pathological conditions in humans. Using immunohistochemical, biochemical, and genetic interaction approaches followed by the detailed analysis of the muscle tissue architecture, we verified Dg interaction with some of the discovered factors. -

Alpha-Actinin-3 R577X
Annals of Applied Sport Science, vol. 4, no. 4, pp. 01-06, Winter 2016 DOI: 10.18869/acadpub.aassjournal.4.4.1 Short Communication www.aassjournal.com www.AESAsport.com ISSN (Online): 2322 – 4479 Received: 20/03/2016 ISSN (Print): 2476–4981 Accepted: 10/06/2016 Alpha-actinin-3 R577X Polymorphism Profile of Turkish Professional Hip-Hop and Latin Dancers 1,2 * 1 1 2 1 1 Korkut Ulucan , Betul Biyik, Sezgin Kapici, Canan Sercan, Oznur Yilmaz, Tunc Catal 1Üsküdar Univerity, Haluk Turksoy Sok. No:14, Altunizade, Üsküdar, İstanbul, Turkey. 2Marmara University, BAsibuyuk Yolu 9/3 MAltepe Saglık Yerleşkesi, MAltepe, Istanbul, Turkey. ABSTRACT Actins are small globular filaments functioning in cell processes like muscle contraction, and stabilized to the sarcomeric Z- discs by actin binding proteins (actinins). One of the important gene coding for actin binding proteins in fast twitch fibers is alpha- actinin- 3 (ACTN3). In this research, we have conducted a gene profile study investigating the genotype and allele distributions of ACTN3 R577X polymorphism in Turkish professional hip- hop and latin dancers and compared them to non-dancers as a control group. 30 professional dancers and non-dancers were recruited for the study. A genotyping procedure was carried out by a newly introduced four-primer PCR methodology. For statistical analysis, the Chi-square test was used to compare data between the groups (p<0,05 evaluated as significant). Numbers and the percentages of dancers were 2 (7%), 21 (70%) and 7(23%) for RR, RX and XX genotypes, respectively. The same numbers and the percentages were 15 (50%), 8 (15%) and 7 (23%) for RR, RX and XX genotypes, respectively, for the controls. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
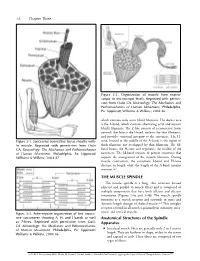
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles.