Effect of Lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
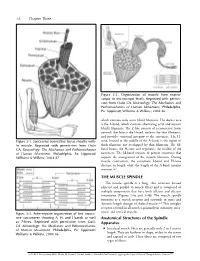
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Spinal Reflexes
Spinal Reflexes Lu Chen, Ph.D. MCB, UC Berkeley 1 Simple reflexes such as stretch reflex require coordinated contraction and relaxation of different muscle groups Categories of Muscle Based on Direction of Motion Flexors Æ reduce the angle of joints Extensors Æ increase the angle of joints Categories of Muscle Based on Movement Agonist Æmuscle that serves to move the joint in the same direction as the studied muscle Antagonist Æ muscle that moves the joint in the opposite direction 2 1 Muscle Spindles •Small encapsulated sensory receptors that have a Intrafusal muscle spindle-like shape and are located within the fibers fleshy part of the muscle •In parallel with the muscle fibers capsule •Does not contribute to the overall contractile Sensory force endings •Mechanoreceptors are activated by stretch of the central region Afferent axons •Due to stretch of the whole muscle Efferent axons (including intrafusal f.) •Due to contraction of the polar regions of Gamma motor the intrafusal fibers endings 3 Muscle Spindles Organization 2 kinds of intrafusal muscle fibers •Nuclear bag fibers (2-3) •Dynamic •Static •Nuclear chain fibers (~5) •Static 2 types of sensory fibers •Ia (primary) - central region of all intrafusal fibers •II (secondary) - adjacent to the central region of static nuclear bag fibers and nuclear chain fibers Intrafusal fibers stretched Sensory ending stretched, (loading the spindle) increase firing Muscle fibers lengthens Sensory ending stretched, (stretched) increase firing Spindle unloaded, Muscle fiber shortens decrease firing 4 2 Muscle Spindles Organization Gamma motor neurons innervate the intrafusal muscle fibers. Activation of Shortening of the polar regions gamma neurons of the intrafusal fibers Stretches the noncontractile Increase firing of the center regions sensory endings Therefore, the gamma motor neurons provide a mechanism for adjusting the sensitivity of the muscle spindles. -

Periostin Is Required for the Maintenance of Muscle Fibers During Muscle Regeneration
International Journal of Molecular Sciences Article Periostin Is Required for the Maintenance of Muscle Fibers during Muscle Regeneration Naoki Ito 1,2,3 , Yuko Miyagoe-Suzuki 2 , Shin’ichi Takeda 2,* and Akira Kudo 3,* 1 Laboratory of Molecular Life Science, Institute of Biomedical Research and Innovation (IBRI), Foundation for Biomedical Research and Innovation at Kobe (FBRI), Kobe 650-0047, Japan; [email protected] 2 National Center of Neurology and Psychiatry, Department of Molecular Therapy, National Institute of Neuroscience, Tokyo 187-8502, Japan; [email protected] 3 Department of Biological Information, Tokyo Institute of Technology, Yokohama 226-8501, Japan * Correspondence: [email protected] (S.T.); [email protected] (A.K.) Abstract: Skeletal muscle regeneration is a well-organized process that requires remodeling of the extracellular matrix (ECM). In this study, we revealed the protective role of periostin, a matricellular protein that binds to several ECM proteins during muscle regeneration. In intact muscle, periostin was localized at the neuromuscular junction, muscle spindle, and myotendinous junction, which are connection sites between muscle fibers and nerves or tendons. During muscle regeneration, periostin exhibited robustly increased expression and localization at the interstitial space. Periostin-null mice showed decreased muscle weight due to the loss of muscle fibers during repeated muscle regeneration. Cultured muscle progenitor cells from periostin-null mice showed no deficiencies in their proliferation, differentiation, and the expression of Pax7, MyoD, and myogenin, suggesting that the loss of muscle Citation: Ito, N.; Miyagoe-Suzuki, Y.; fibers in periostin-null mice was not due to the impaired function of muscle stem/progenitor cells. -

Stretch-Induced Contraction of Intrafusal Muscle in Cat Muscle Spindle1
0270~6474/81/0110-1069$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 1, No. 10, pp. 1069-1074 Printed in U.S.A. October 1981 STRETCH-INDUCED CONTRACTION OF INTRAFUSAL MUSCLE IN CAT MUSCLE SPINDLE1 R. E. POPPELE*x ’ AND D. C. QUICK+ *Laboratory of Neurophysiology and *Departments of Neurology and Anatomy, University of Minnesota, Minneapolis, Minnesota 55455 Abstract Measurements of tension, stiffness, and sarcomere length of intrafusal muscle during ramp stretch of isolated muscle spindles have revealed a stretch-induced contraction of the bag1 fiber. This behavior can account for the very high sensitivity of primary endings to stretch as well as the enhanced sensitivity evoked by dynamic fusimotor stimulation. An intriguing aspect of mammalian muscle spindle Materials and Methods behavior is the very high sensitivity of the primary ending Spindle afferent discharge was recorded from dorsal compared to that of the secondary ending in the same root filaments of anesthetized cats (pentobarbital so- receptor. There are at least two morphological differ- dium, Nembutal, Abbott Laboratories, 35 mg/kg). The ences between primary and secondary endings that might hindlimb was denervated except for the medial gas- account for this difference. The primary ending is formed trocnemius, and ventral roots’L5 through Sl were cut. on the intrafusal bundle in a region called the equator Activity of single muscle spindle receptors was identified where there are very few myofilaments. The secondary in the usual manner (e.g., Poppele, 1981). Mechanical endings are formed in the juxtaequatorial region where measurements were made on isolated spindles obtained there are abundant myofilaments. -

Muscle Spindle Modeling - a Tutorial
Faculty of Electrical Engineering Mechatronics Engineering Muscle Spindle Modeling - A Tutorial Professor: Dr. Mehdi Delrobaei Student Name: Sadaf Yari January-2019 Muscle Spindle Every day we move around endlessly, walking, exercising, etc. We perform these tasks without thinking about it. In fact, for the human body to make the simplest motion, such as lifting an arm, requires the human brain to perform a dozen calculations and control many complex procedures. Some muscles have to contract while others to expand. The final goal is reached through careful control of the muscles via feedback which provides the brain with information on the current situation of the different parts of the body. One such feedback mechanism is proprioception which contains information on the current location of body parts and the situation they are in. This is made possible by sensors, called proprioceptors, located in the muscles. Examples of proprioceptors include the muscle spindle and the Golgi tendon organ. The former provides length information of the muscle and the latter detects changes in the muscle stretch. Such information is useful for the brain when attempting to control the motion of the body parts. In this tutorial we will focus on the muscle spindle. First, a detailed anatomical and physiological description of the muscle spindle’s structure and function is given. In this part it is explained where exactly the muscle spindle is located and what it does. Then, a mathematical model, which is currently accepted generally, is discussed. What is muscle spindle? Muscles are the organs that cause movement in our body. Each motion in the body, wether volunatry or not, is caused by the contraction or release of a muscle. -

The Somatic Nervous System Mimi Jakoi, Phd Jennifer Carbrey, Phd
Introductory Human Physiology ©copyright Jennifer Carbrey & Emma Jakoi The Somatic Nervous System Mimi Jakoi, PhD Jennifer Carbrey, PhD The underlined headings correspond to the two Somatic Nervous system videos. 1. Introduction and structure The efferent portion of the peripheral nervous system consists of the somatic nervous system and the autonomic nervous system. The autonomic nervous system controls the function of glands, smooth muscle, cardiac muscle, and the neurons of the GI tract. It is composed of two neurons in series that can either excite or inhibit the target organ. In contrast, the somatic nervous system contains single neurons that excite skeletal muscles. The movements controlled by the somatic nervous system can be voluntary or involuntary (reflexes). Motor Unit The axons of motor neurons are myelinated and have large diameters for fast conduction of action potentials. As the axon approaches a skeletal muscle fiber (muscle cell) it usually branches to form synapses with anywhere from three to one thousand muscle fibers. However, each muscle fiber is usually innervated by only a single neuron. A motor unit consists of a neuron and all of the muscle fibers it innervates. A single neuron innervates fibers from only one muscle and the innervated muscle fibers are usually spread throughout the muscle. The portion of the skeletal muscle fiber plasma membrane that synapses with the motor neuron axon is called the motor end plate. Once an action potential arrives at the axon terminal, the depolarization of the membrane opens voltage-gated calcium channels (Fig. 1). An increase in intracellular calcium at the terminal causes release of acetylcholine vesicles into the neuromuscular junction. -

MUSCLE STRETCH REFLEX: Components and Process Description
MUSCLE STRETCH REFLEX: Components and Process Description Introduction The muscle stretch reflex is an unconscious action caused by the collaboration between a person’s nervous and muscular systems. The reflex acts to prevent damage to muscles and maintain sensory input to the central nervous system. Often, these reflexes are tested during check-ups to make sure there are no problems with the patient’s nervous and muscular systems. The reflex happens when a muscle is stretched and causes an unconscious contraction of the stretched muscles to prevent injury. To describe the process, this description will be looking at the knee jerk reflex and will explain how muscle spindles regulate such a reflex. Muscle Spindle Location and Components Muscle spindles are arranged within whole muscle; parallel to the muscle fibers. As seen in Figure 1, there are many components involved in muscle stretch reflex. • Extrafusal muscle fibers: These are the normal, contractile muscle fibers found in skeletal muscles. These fibers are innervated by alpha motor neurons (not shown in Figure 1). • Muscle spindle: This is the Figure 1: Muscle Spindle sensory and regulatory organ involved in the muscle stretch reflex. It is arranged within muscles; parallel to the muscle fibers. There are various components that make up a muscle spindle. These components include: o The central region: This is the middle part of the muscle spindle. The central region lacks myofibrils and is noncontractile. This region contains the ends of a sensory afferent neuron. o The sensory afferent neuron: This is a tonically active (always firing action potentials) sensory neuron that relays information from the muscle spindle to the central nervous system. -

Histological Aspects of Skeletal Muscle Fibers Splitting of C57BL/6Ncrl Mice
Physiol. Res. 69: 291-296, 2020 https://doi.org/10.33549/physiolres.934245 Histological Aspects of Skeletal Muscle Fibers Splitting of C57BL/6NCrl Mice Peter MAKOVICKÝ1, Pavol MAKOVICKÝ2 1Czech Centre for Phenogenomics, Institute of Molecular Genetic of the Czech Academy of Sciences, Vestec, Czech Republic. 2Department of Biology, Faculty of Education, J. Selye University, Komarno, Slovak Republic Received July 1, 2019 Accepted November 28, 2019 Epub Ahead of Print March 23, 2020 Summary orchestrated process and the factors that impact skeletal The objective of the current study is to present data on the muscle structure, function and regeneration are of great splitting of skeletal muscle fibers in C57BL/6NCrl mice. Skeletal importance and interest not only scientifically but also muscles (m. rectus femoris (m. quadriceps femoris)) from 500 clinically. This means, skeletal muscles regeneration is in (250 ♀ and 250 ♂) C57BL/6NCrl mice in the 16th week of life the interest of medical research (Kinter and Sinnreich were sampled during autopsy and afterwards standardly 2014, Liu et al. 2018). Today skeletal muscle histologically processed. Results show spontaneous skeletal regeneration is studied at a high level including injury, muscle fiber splitting which is followed by skeletal muscle fiber development, factors contributing to regeneration, regeneration. One solitary skeletal muscle fiber is split, or is in satellite cells, stem cells, the role of secreted factors and contact with few localized splitting skeletal muscle fibers. Part of extracellular matrix remodelling (Baghdadi and the split skeletal muscular fiber is phagocytosed, but the Tajbakhsh 2018, Liu et al. 2019, Wosczyna et al. 2019). remaining skeletal muscular fiber splits are merged into one The results are important for applied research and regenerating skeletal muscle fiber.