VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1933.Full.Pdf
0270.6474/84/0408-1933$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 8, pp. 1933-1943 Printed in U.S.A. August 1984 PARTIAL PURIFICATION AND FUNCTIONAL IDENTIFICATION OF A CALMODULIN-ACTIVATED, ADENOSINE 5’-TRIPHOSPHATE-DEPENDENT CALCIUM PUMP FROM SYNAPTIC PLASMA MEMBRANES1 DIANE M. PAPAZIAN,* HANNAH RAHAMIMOFF,$ AND STANLEY M. GOLDIN§’ Departments of *Biological Chemistry and $Pharmacology, Harvard Medical School, Boston, Massachusetts 02115 and *Department of Biochemistry, Hadassah Medical School, Hebrew University, Jerusalem, Israel Received July 18, 1983; Revised February 27, 1984; Accepted February 28, 1984 Abstract Synaptic plasma membranes isolated from rat brain contain a calmodulin-activated Ca2+ pump. It has been purified 80- to 160-fold by solubilization with Triton X-100 and affinity chromatography on a calmodulin- Sepharose 4B column. After reconstitution into phospholipid vesicles, the affinity-purified pump efficiently catalyzed ATP dependent Ca2+ transport, which was activated 7- to g-fold by calmodulin. The major protein component of the affinity-purified preparation had a M, = 140,000; it was virtually the only band visualized on a Coomassie blue-stained SDS polyacrylamide gel. It has been identified as the Ca”’ pump by two functional criteria. First, it was phosphorylated by [y-““P]ATP in a Ca’+-dependent manner; the phosphorylated protein had the chemical reactivity of an acyl phosphate, characteristic of the phosphorylated intermediates of ion-transporting ATPases. Second, the protein was enriched by transport-specific fractiona- tion, a density gradient procedure which uses the transport properties of the reconstituted Ca2+ pump as a physical tool for its purification. -

Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections Acta Scientiae Veterinariae, Vol
Acta Scientiae Veterinariae ISSN: 1678-0345 [email protected] Universidade Federal do Rio Grande do Sul Brasil Tejero, Felix; Arias-Mota, Lourdes Lorena; Roschman-González, Antonio; Aso, Pedro María; Finol, Héctor José Trypanosoma evansi: Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections Acta Scientiae Veterinariae, vol. 38, núm. 3, 2010, pp. 279-285 Universidade Federal do Rio Grande do Sul Porto Alegre, Brasil Available in: http://www.redalyc.org/articulo.oa?id=289021902008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Acta Scientiae Veterinariae. 38(3): 279-285, 2010. ORIGINAL ARTICLE ISSN 1679-9216 (Online) Pub. 910 Trypanosoma evansi: Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections* Felix Tejero1, Lourdes Lorena Arias-Mota1, Antonio Roschman-González2, Pedro María Aso3 & Héctor José Finol2 ABSTRACT Background: Trypanosoma evansi is the etiologic agent of the equine trypanosomosis, a disease related to the detriment of the extensive bovine farming in the Venezuelan grasslands. Even though macroscopic pathologies such as anemia, pale mucosa, icteric tissues, generalized edema, splenomegaly, liver and renal hypertrophy, abortion, anoestrus, emaciation, lymphadenopathies, striated muscle atrophy as well as epicardiac and endocardiac hemorrhages have been described for infections with the agent, no reports of any heart ultrastructural change in experimental or natural infections induced by Venezuelan T. evansi isolates are available. So, a transmission electron microscopic approach to the problem was needed. This work describes cell features of the cardiac myocyte and the cardiac microvasculature ultrastructure in mice experimentally infected with an equine local isolate of T. -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -

Desmin- Cytoskeleton Marker Cat#: ET1606-30
rev. 04/13/16 Desmin- Cytoskeleton Marker Cat#: ET1606-30 Prod uct Type: R ecombinant r abbit mono clonal IgG, primary antibodies Species reactivity: Human, Mouse, Rat, Zebra fish Applications: WB, ICC/IF, IHC, FC Molecular Wt.: 53 kDa Description: Cytoskeletal intermediate filaments (IFs) constitute a diverse group of proteins that are expressed in a highly tissue - specific manner. IFs are constructed from two - chain α -helical coiled-coil molecules arranged on an imperfect helical Fig1: Western blot analysis of Desmin on lattice, and have been widely used as markers for distinguishing different lysates using anti-Desmin antibody at individual cell types within a tissue and identifying the origins 1/1,000 dilution. of metastatic tumors. Vimentin is an IF general marker of cells Positive control: originating in the mesenchyme. Vimentin and Desmin, a related class III IF, are both expressed during skeletal muscle development. Lane 1: Human skeletal muscle Desmin, a 469 amino acid protein found near the Z line in sarcomeres, Lane 2: Mouse heart is expressed more frequently in adult differentiated state tissues. Desmin makes up attachments between the terminal Z-disc and membrane-associated proteins to form a force-transmitting system. Mutations in the gene encoding for Desmin are associated with adult-onset skeletal myopathy, sporadic disease and mild cardiac involvement. Immunogen: Recombinant protein. Positive control: Fig2: ICC staining Desmin in C2C12 cells (green). C2C12, human uterus tissue, mouse bladder tissue, mouse heart The nuclear counter stain is DAPI (blue). Cells tissue, mouse skeletal muscle tissue. were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

The Muscular System
THE MUSCULAR SYSTEM COMPILED BY HOWIE BAUM 1 Muscles make up the bulk of the body and account for 1/3 of its weight.!! Blood vessels and nerves run to every muscle, helping control and regulate each muscle’s function. The muscular system creates body heat and also moves the: Bones of the Skeletal system Food through Digestive system Blood through the Circulatory system Fluids through the Excretory system MUSCLE TISSUE The body has 3 main types of muscle tissue 1) Skeletal, 2) Smooth, and 3) Cardiac SKELETAL MUSCLE SMOOTH MUSCLE CARDIAC MUSCLE Skeletal muscles attach to and move bones by contracting and relaxing in response to voluntary messages from the nervous system. Skeletal muscle tissue is composed of long cells called muscle fibers that have a striated appearance. Muscle fibers are organized into bundles supplied by blood vessels and innervated by motor neurons. Muscle structure Skeletal (striated or voluntary) muscle consists of densely packed groups of hugely elongated cells known as myofibers. These are grouped into bundles (fascicles). A typical myofiber is 2–3 centimeters ( 3/4–1 1/5 in) long and 0.05millimeters (1/500 inch) in diameter and is composed of narrower structures – myofibrils. These contain thick and thin myofilaments made up mainly of the proteins actin and myosin. Numerous capillaries keep the muscle supplied with the oxygen and glucose needed to fuel contraction. Skeletal Muscles • Skeletal muscles attach to bones by tendons (connective tissue) and enable movement. • Skeletal muscles are mostly voluntary Feel the back of your ankle to feel your Achilles tendon - the largest tendon in your body. -

Muscle Histology
Muscle Histology Dr. Heba Kalbouneh Functions of muscle tissue ▪ Movement ▪ Maintenance of posture ▪ Joint stabilization ▪ Heat generation Types of Muscle Tissue ▪ Skeletal muscle ▪ Cardiac muscle ▪ Smooth muscle Types of Muscle Tissue Skeletal •Attach to and move skeleton •40% of body weight •Fibers = multinucleate cells (embryonic cells fuse) •Cells with obvious striations •Contractions are voluntary Cardiac: only in the wall of the heart •Cells are striated •Contractions are involuntary (not voluntary) Smooth: walls of hollow organs •Lack striations •Contractions are involuntary (not voluntary) Similarities… ▪ Their cells are called fibers because they are elongated ▪ Contraction depends on myofilaments ▪ Actin ▪ Myosin ▪ Plasma membrane is called sarcolemma ▪ Sarcos = flesh ▪ Lemma = sheath SKELETAL MUSCLES Epimysium: surrounds whole muscle Endomysium is around each muscle fiber Perimysium is around fascicle = muscle cell= myofiber Skeletal muscle This big cylinder is a fiber: a cell ▪ Fibers (each is one cell) have striations ▪ Myofibrils are organelles of the cell: these are made -an organelle up of myofilaments ▪ Sarcomere ▪ Basic unit of contraction ▪ Myofibrils are long rows of repeating sarcomeres ▪ Boundaries: Z discs (or lines) Sarcomere M line provides an attachment to myosin filaments Z line provides an attachment to actin filaments A band is the darker band of the myofibril containing myosin filaments H band is the lighter section in the middle of the A band where only myosin is present I band is the lighter band containing -

(7E) Powerpoint Lecture Outline Chapter 8: Control of Movement
Carlson (7e) PowerPoint Lecture Outline Chapter 8: Control of Movement This multimedia product and its contents are protected under copyright law. The following are prohibited by law: •any public performance or display, including transmission of any image over a network; •preparation of any derivative work, including extraction, in whole or in part, of any images; •any rental, lease, or lending of the program. Copyright 2001 by Allyn & Bacon Skeletal Muscle n Movements of our body are accomplished by contraction of the skeletal muscles l Flexion: contraction of a flexor muscle draws in a limb l Extension: contraction of extensor muscle n Skeletal muscle fibers have a striated appearance n Skeletal muscle is composed of two fiber types: l Extrafusal: innervated by alpha-motoneurons from the spinal cord: exert force l Intrafusal: sensory fibers that detect stretch of the muscle u Afferent fibers: report length of intrafusal: when stretched, the fibers stimulate the alpha-neuron that innervates the muscle fiber: maintains muscle tone u Efferent fibers: contraction adjusts sensitivity of afferent fibers. 8.2 Copyright 2001 by Allyn & Bacon Skeletal Muscle Anatomy n Each muscle fiber consists of a bundle of myofibrils l Each myofibril is made up of overlapping strands of actin and myosin l During a muscle twitch, the myosin filaments move relative to the actin filaments, thereby shortening the muscle fiber 8.3 Copyright 2001 by Allyn & Bacon Neuromuscular Junction n The neuromuscular junction is the synapse formed between an alpha motor neuron -

Tissue Engineered Myelination and the Stretch Reflex Arc Sensory Circuit: Defined Medium Ormulation,F Interface Design and Microfabrication
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2009 Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium ormulation,F Interface Design And Microfabrication John Rumsey University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rumsey, John, "Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium Formulation, Interface Design And Microfabrication" (2009). Electronic Theses and Dissertations, 2004-2019. 3826. https://stars.library.ucf.edu/etd/3826 TISSUE ENGINEERED MYELINATION AND THE STRETCH REFLEX ARC SENSORY CIRCUIT: DEFINED MEDIUM FORMULATION, INTERFACE DESIGN AND MICROFABRICATION by JOHN WAYNE RUMSEY B.S. University of Florida, 2001 M.S. University of Central Florida, 2004 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Fall Term 2009 Major Professor: James J. Hickman ABSTRACT The overall focus of this research project was to develop an in vitro tissue- engineered system that accurately reproduced the physiology of the sensory elements of the stretch reflex arc as well as engineer the myelination of neurons in the systems. In order to achieve this goal we hypothesized that myelinating culture systems, intrafusal muscle fibers and the sensory circuit of the stretch reflex arc could be bioengineered using serum-free medium formulations, growth substrate interface design and microfabrication technology. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
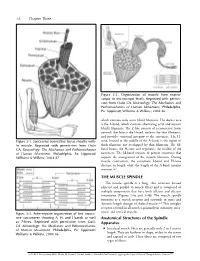
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Be Able to Describe a Stretch Reflex
be able to describe a stretch reflex .(1) (2) Define muscle tone (3) be able to explain what is muscle tone (4) describe the structure , innervations and function of the muscle spindle . (5) explain what is meant by static and dynamic stretch reflex . (6) describe the spinal and supraspinal regulation of the stretch reflex . (7) describe the inverse stretch reflex and its function Stretch reflex: Whenever a muscle is stretched suddenly, excitation of the muscle spindle causes reflex contraction of the large muscle fiber (See the pictures). It is deep and monosynaptic reflex. Stretch response is produced by co-activation of alpha & gamma motor neurons. But it is maintained mainly by the tonic ( continuous) discharge of Gamma Efferent neurons. Muscle spindle: It is a sensory receptors which are distributed throughout the muscle and it sends information to nervous system about muscle length or rate of change in muscle length. - Each spindle is built around 3-12 intrafusal muscle fibers. - Muscle spindles are parallel to extrafusal muscle fibers and they are attached to them or to the tendon. Types of Intrafusal fibers Each intrafusal fiber consists of: (1) Central non-contractile area (receptor 2) Nuclear chain fibers: area). thinner and shorter 1) Nuclear bag fibers: than nuclear bag fibers (2) Peripheral contractile area. contain many nuclei in , and have one line of a dilated central area ( “ nuclei spread in a chain bag ” ) . Typically there along the receptor area are 2 nuclear bag fibers . There are 4 – 9 per spindle . nuclear chain -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body.