Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Of 100 1. the Golgi Tendon Organ Is an Essential Component of Static
1. The Golgi tendon organ is an essential component of static stretching because it A. increases muscle spindle activity in a tight muscle. Rationale A. Golgi tendon organs decrease muscle spindle activity. B. prevents muscles from stretching too far or too fast. Rationale B. Muscle spindles prevent muscles from stretching too far or too fast. C. increases contraction rate in muscle fibers. Rationale C. Holding a stretch creates tension in the muscle, which stimulates the Golgi tendon organ, causes relaxation of an overactive muscle, and allows optimal lengthening of tissue. D. prevents muscle from being placed under excessive tension.(correct) Rationale D. The Golgi tendon organ prevents muscles from being placed under excessive tension (autogenic inhibition). Question Name 1-1 Certification Thinking Skills Foundational Thinking Current Forms 2011 Practice A Primary Reference Chapter 7, Task 1A1 Page 1 of 100 2. Which of the following is the correct force-couple relationship that allows for the upward rotation of the scapula? A. Longus capitus and brachialis Rationale A. The longus capitus concentrically accelerates cervical flexion and lateral flexion, while the brachialis concentrically accelerates elbow flexion. B. Rhomboid minor and anterior scalenes Rationale B. The rhomboid minor concentrically accelerates scapular retraction and downward rotation, while the anterior scalenes concentrically accelerates cervical flexion, rotation, and lateral flexion. C. Sternocleidomastoid and longus coli Rationale C. The sternocleidomastoid concentrically accelerates cervical flexion, rotation, and lateral flexion while the longus coli concentrically accelerate cervical flexion, lateral flexion, and ipsilateral rotation. D. Upper trapezius and lower portions of the serratus anterior (correct) Rationale D. The upper trapezius and the lower portion of the serratus anterior are muscle groups that move together to produce upward rotation of the scapula. -

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Fine Structure of the Receptors at the Myotendinous Junction of Human Extraocular Muscles
Histol Histopath (1 988) 3: 103-113 Histology and Fine structure of the receptors at the myotendinous junction of human extraocular muscles A. Sodii, M. Corsii, M.S. Faussone Pellegrini2and G. Salvii 'Eye Clinic, Chair of Physiopathological Optics and Departrnent of Hurnan Anatorny and Histology, Section of Histology, University of Florence, ltaly Summary. The myotendinous junction of the human lntroduction extraocular muscles was studied by electron microscopy. Some peculiar receptorial structures have been found in The proprioceptors known as tendon organs were first the majority of the samples examined. These structures identified by Golgi in 1880 in skeletal muscles. They were are very small and consist of 1) the terminal portion of first described at electron microscope level by Merrillees one muscle fibre, 2) the tendon into which it inserts and in 1962 and later by other authors (Schoultz and Swett, y), within the tendon, a rich nerve arborization, whose 1972, 1974; Barker, 1974: Zelena and Soukup, 1977; branches are always very close to the rnuscle component. Soukup and Zelena, 1985; Ovalle and Dow, 1983). In the Only one discontinuous layer, made up of tlat cells. extraocular muscles (EOM) the presence of tendon which lack a basa1 lamina and often show pinocytotic organs was first excluded by Golgi himself, but further vesicles, encapsules every musculo-tendinous complex. investigations (Dogiel. 1906: Loffredo-Sampaolo, 1952; The tendinous component consists of amorphous ground Bonavolonta, 1956, 1958) led to their identification and substance of different electron density. of collagen and description at light microscopy level in several animal elastic fibres and is divided in compartments by ramified species. -

Neural Control and Neuromodulationof Lower Urinary
Neural Control and Neuromodulation of Lower Urinary Tract Function William C. de Groat University of Pittsburgh Topics • Anatomy and functions of the lower urinary tract • Peripheral innervation (efferent and afferent nerves) • Central neural control of the lower urinary tract • Lower urinary tract dysfunction • Treatment of dysfunction (neuromodulation) • Research opportunities Anatomy and Functions of the Lower Urinary Tract Functions Two Types of Voiding 1. Urine storage - Reservoir: Bladder INVOLUNTARY (Reflex) (infant & fetus) Defect in 2. Urine release Maturation Maturation - Outlet: Urethra INVOLUNTARY THERAPY VOLUNTARY (Reflex) (adult) (adult) Bladder Parkinson’s, MS, stroke, brain tumors, spinal cord injury, Urethra aging, cystitis Lower Urinary Tract Innervation Two Types of Visceral Afferent Neurons: Bladder & Bowel Aδ-fibers responsible for normal bladder sensations C-fibers contribute to urgency, frequency and incontinence Afferent Sensitivity may be Influenced by Substances Released from the Urothelium Aδ fiber C fiber X CNS Urothelium The Bladder U rothelium Glycosaminoglycan Layer Uroplakin Plaques and Discoidal Vesicles r=_zo_"_"l_a_o_cc-lu---.dens } Umbrella Cell Stratum Intermediate Cell Stratum } Basal Cell Stratum Afferent Nerve fiber Urothelial-Afferent Interactions ( I ( I ( I ( ' ( I ( I ( I Urothelium ( I ( 0 I I I AChR ATP, NO, NKA, ACh, NGF ~erves ' Efferent ' nerves nerves Interaction of Sensory Pathways of Multiple Pelvic Organs Convergent Dichotomizing Branch Bladder point Colon Somatic and Visceral Afferent -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
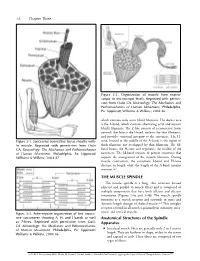
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Effect of Lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’
0270.6474/85/0507-1881$02.00/O The Journal of Neurowence CopyrIght 0 Society for Neuroscience Vol. 5, No. 7, pp. 1881-1885 Printed in U.S.A. July 1985 Effect of lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’ R. E. POPPELE*V2 AND D. c. QUICK* * Laboratory of Neurophysiology and $ Department of Anatomy, University of Minnesota, Minneapolis, Minnesota 55455 Abstract Materials and Methods Spindles were dissected free from tenuissimus muscles taken from anes- Sensitivity differences between primary and secondary thetized cats (pentobarbttal sodium, Nembutal, Abbott Laboratories, 35 mg/ endings of mammalian muscle spindles under various con- kg, or ketamine hydrochloride, Parke, Davis, 20 mg/kg). The isolated recep- ditions of stretch and fusimotor activation may be due to tor, together with about 1 cm of nerve, was mounted in a small chamber by differences in their respective mechanoelectric transducers tying each pole (near the capsule sleeve) to a small tungsten wire shaft or to mechanical properties of the intrafusal muscle support- connected to a servo-controlled Ling vibrator (model 108). The chamber was ing those endings. This study of isolated cat muscle spindles continuously perfused with oxygenated, modified Krebs’ solution (Poppele et al., 1979). The nerve was drawn onto a pair of electrodes in an adjacent examines the strain in individual intrafusal muscle fibers chamber containtng a high density fluorocarbon compound (FC-80, 3M Co.). resulting from stretch and fusimotor stimulation. The degree The entire assembly was mounted on a Zeiss photomicroscope equipped of local stretch occurring at the sensory endings under these with tine camera and Nomarski optics (see Poppele et al., 1979, and Poppele conditions was measured. -

Spinal Reflexes
Spinal Reflexes Lu Chen, Ph.D. MCB, UC Berkeley 1 Simple reflexes such as stretch reflex require coordinated contraction and relaxation of different muscle groups Categories of Muscle Based on Direction of Motion Flexors Æ reduce the angle of joints Extensors Æ increase the angle of joints Categories of Muscle Based on Movement Agonist Æmuscle that serves to move the joint in the same direction as the studied muscle Antagonist Æ muscle that moves the joint in the opposite direction 2 1 Muscle Spindles •Small encapsulated sensory receptors that have a Intrafusal muscle spindle-like shape and are located within the fibers fleshy part of the muscle •In parallel with the muscle fibers capsule •Does not contribute to the overall contractile Sensory force endings •Mechanoreceptors are activated by stretch of the central region Afferent axons •Due to stretch of the whole muscle Efferent axons (including intrafusal f.) •Due to contraction of the polar regions of Gamma motor the intrafusal fibers endings 3 Muscle Spindles Organization 2 kinds of intrafusal muscle fibers •Nuclear bag fibers (2-3) •Dynamic •Static •Nuclear chain fibers (~5) •Static 2 types of sensory fibers •Ia (primary) - central region of all intrafusal fibers •II (secondary) - adjacent to the central region of static nuclear bag fibers and nuclear chain fibers Intrafusal fibers stretched Sensory ending stretched, (loading the spindle) increase firing Muscle fibers lengthens Sensory ending stretched, (stretched) increase firing Spindle unloaded, Muscle fiber shortens decrease firing 4 2 Muscle Spindles Organization Gamma motor neurons innervate the intrafusal muscle fibers. Activation of Shortening of the polar regions gamma neurons of the intrafusal fibers Stretches the noncontractile Increase firing of the center regions sensory endings Therefore, the gamma motor neurons provide a mechanism for adjusting the sensitivity of the muscle spindles. -

Neural Control of Human Movement
97818_ch19.qxd 8/4/09 4:16 PM Page 376 CHAPTER 19 Neural Control of Human Movement CHAPTER OBJECTIVES ➤ Draw the major structural components of the ➤ Outline motor unit facilitation and inhibition and brain, including the four lobes of the cerebral the contribution of each to exercise performance cortex and responsiveness to resistance training ➤ Discuss specific pyramidal and extrapyramidal ➤ Discuss variations in twitch characteristics, resist- tract functions ance to fatigue, and tension development in the different motor unit categories ➤ Diagram the anterior motor neuron and discuss its role in human movement ➤ Describe mechanisms that adjust force of muscle action along the continuum from slight to ➤ Draw and label the basic components of a maximum reflex arc ➤ Define fatigue and discuss factors that act and ➤ Define the terms (1) motor unit, (2) neuromuscular interact to induce neuromuscular fatigue junction, and (3) autonomic nervous system ➤ List and describe functions of the proprioceptors ➤ Summarize the events in motor unit excitation within joints, muscles, and tendons prior to muscle action 376 97818_ch19.qxd 8/4/09 4:16 PM Page 377 CHAPTER 19 Neural Control of Human Movement 377 The effective application of force during complex learned Brainstem movements (e.g., tennis serve, shot put, golf swing) depends on a series of coordinated neuromuscular patterns, not just on The medulla, pons, and midbrain compose the brain- muscle strength. The neural circuitry in the brain, spinal cord, stem. The medulla, located immediately above the spinal and periphery functions somewhat similar to a sophisticated cord, extends into the pons and serves as a bridge between computer network. -

Analysis of Proprioceptive Sensory Innervation of the Mouse Soleus: a Whole-Mount Muscle Approach
Wright State University CORE Scholar Neuroscience, Cell Biology & Physiology Faculty Publications Neuroscience, Cell Biology & Physiology 1-25-2017 Analysis of Proprioceptive Sensory Innervation of the Mouse Soleus: A Whole-Mount Muscle Approach Martha Jean Sonner Wright State University Marie C. Walters Wright State University David R. Ladle Wright State University - Main Campus, [email protected] Follow this and additional works at: https://corescholar.libraries.wright.edu/ncbp Part of the Medical Cell Biology Commons, Medical Neurobiology Commons, Medical Physiology Commons, Neurosciences Commons, and the Physiological Processes Commons Repository Citation Sonner, M. J., Walters, M. C., & Ladle, D. R. (2017). Analysis of Proprioceptive Sensory Innervation of the Mouse Soleus: A Whole-Mount Muscle Approach. PLOS ONE, 12 (1), e0170751. https://corescholar.libraries.wright.edu/ncbp/1104 This Article is brought to you for free and open access by the Neuroscience, Cell Biology & Physiology at CORE Scholar. It has been accepted for inclusion in Neuroscience, Cell Biology & Physiology Faculty Publications by an authorized administrator of CORE Scholar. For more information, please contact [email protected]. RESEARCH ARTICLE Analysis of Proprioceptive Sensory Innervation of the Mouse Soleus: A Whole- Mount Muscle Approach Martha J. Sonner, Marie C. Walters, David R. Ladle* Department of Neuroscience, Cell Biology, and Physiology, Wright State University, Dayton, Ohio, United States of America * [email protected] a1111111111 Abstract a1111111111 a1111111111 Muscle proprioceptive afferents provide feedback critical for successful execution of motor a1111111111 a1111111111 tasks via specialized mechanoreceptors housed within skeletal muscles: muscle spindles, supplied by group Ia and group II afferents, and Golgi tendon organs, supplied by group Ib afferents. -

Stretch Reflex & Golgi Tendon Reflex
NeuroPsychiatry Block Stretch Reflex & Golgi Tendon Reflex By Laiche Djouhri, PhD Dept. of Physiology Email: [email protected] Ext:71044 NeuroPsychiatry Block Chapter 55 Motor Functions of the Spinal Cord, The cord Reflexes (Guyton & Hall) Chapter 3 Neurophysiology (Linda Costanzo) 2 Objectives By the end of this lecture students are expected to: . Describe the components of stretch reflex and Golgi tendon reflex . Differentiate between the functions of muscles spindles and Golgi tendon organ . Explain the roles of alpha and gamma motor neurons in the stretch reflex . Discuss the spinal and supraspinal regulation 10of/6/2016 the stretch reflex 3 What is a Stretch Reflex? . It is a monosynaptic reflex (also known as myotatic reflex) . Is a reflex contraction of muscle resulting from stimulation of the muscle spindle (MS) by stretching the whole muscle . Muscle spindle is the sensory receptor that detects change in muscle length . The classic example of the stretch reflex is the patellar-tendon or knee jerk reflex. What is the significance of stretch reflexes? . They help maintain a normal posture . They function to oppose sudden changes in muscle length 4 10/6/2016 Components of the Stretch Reflex Arc Stretch reflex is a deep Figure 55.5 monosynaptic reflex and its components are: 1. Sensory receptor (muscle spindles) 2. Sensory neuron (group Ia and group II afferents) 3. Integrating center (spinal cord) 4. Motor neurons (α- and γ- spinal motor neurons) 5. Effector (the same muscle This reflex is the simplest; it involves (homonymous) of muscle only 2 neurons & one synapse, spindles) Structure of Muscle Spindles-1 .