Stretch-Induced Contraction of Intrafusal Muscle in Cat Muscle Spindle1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections Acta Scientiae Veterinariae, Vol
Acta Scientiae Veterinariae ISSN: 1678-0345 [email protected] Universidade Federal do Rio Grande do Sul Brasil Tejero, Felix; Arias-Mota, Lourdes Lorena; Roschman-González, Antonio; Aso, Pedro María; Finol, Héctor José Trypanosoma evansi: Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections Acta Scientiae Veterinariae, vol. 38, núm. 3, 2010, pp. 279-285 Universidade Federal do Rio Grande do Sul Porto Alegre, Brasil Available in: http://www.redalyc.org/articulo.oa?id=289021902008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Acta Scientiae Veterinariae. 38(3): 279-285, 2010. ORIGINAL ARTICLE ISSN 1679-9216 (Online) Pub. 910 Trypanosoma evansi: Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections* Felix Tejero1, Lourdes Lorena Arias-Mota1, Antonio Roschman-González2, Pedro María Aso3 & Héctor José Finol2 ABSTRACT Background: Trypanosoma evansi is the etiologic agent of the equine trypanosomosis, a disease related to the detriment of the extensive bovine farming in the Venezuelan grasslands. Even though macroscopic pathologies such as anemia, pale mucosa, icteric tissues, generalized edema, splenomegaly, liver and renal hypertrophy, abortion, anoestrus, emaciation, lymphadenopathies, striated muscle atrophy as well as epicardiac and endocardiac hemorrhages have been described for infections with the agent, no reports of any heart ultrastructural change in experimental or natural infections induced by Venezuelan T. evansi isolates are available. So, a transmission electron microscopic approach to the problem was needed. This work describes cell features of the cardiac myocyte and the cardiac microvasculature ultrastructure in mice experimentally infected with an equine local isolate of T. -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -

(7E) Powerpoint Lecture Outline Chapter 8: Control of Movement
Carlson (7e) PowerPoint Lecture Outline Chapter 8: Control of Movement This multimedia product and its contents are protected under copyright law. The following are prohibited by law: •any public performance or display, including transmission of any image over a network; •preparation of any derivative work, including extraction, in whole or in part, of any images; •any rental, lease, or lending of the program. Copyright 2001 by Allyn & Bacon Skeletal Muscle n Movements of our body are accomplished by contraction of the skeletal muscles l Flexion: contraction of a flexor muscle draws in a limb l Extension: contraction of extensor muscle n Skeletal muscle fibers have a striated appearance n Skeletal muscle is composed of two fiber types: l Extrafusal: innervated by alpha-motoneurons from the spinal cord: exert force l Intrafusal: sensory fibers that detect stretch of the muscle u Afferent fibers: report length of intrafusal: when stretched, the fibers stimulate the alpha-neuron that innervates the muscle fiber: maintains muscle tone u Efferent fibers: contraction adjusts sensitivity of afferent fibers. 8.2 Copyright 2001 by Allyn & Bacon Skeletal Muscle Anatomy n Each muscle fiber consists of a bundle of myofibrils l Each myofibril is made up of overlapping strands of actin and myosin l During a muscle twitch, the myosin filaments move relative to the actin filaments, thereby shortening the muscle fiber 8.3 Copyright 2001 by Allyn & Bacon Neuromuscular Junction n The neuromuscular junction is the synapse formed between an alpha motor neuron -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
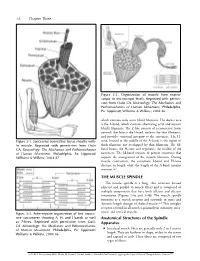
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Effect of Lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’
0270.6474/85/0507-1881$02.00/O The Journal of Neurowence CopyrIght 0 Society for Neuroscience Vol. 5, No. 7, pp. 1881-1885 Printed in U.S.A. July 1985 Effect of lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’ R. E. POPPELE*V2 AND D. c. QUICK* * Laboratory of Neurophysiology and $ Department of Anatomy, University of Minnesota, Minneapolis, Minnesota 55455 Abstract Materials and Methods Spindles were dissected free from tenuissimus muscles taken from anes- Sensitivity differences between primary and secondary thetized cats (pentobarbttal sodium, Nembutal, Abbott Laboratories, 35 mg/ endings of mammalian muscle spindles under various con- kg, or ketamine hydrochloride, Parke, Davis, 20 mg/kg). The isolated recep- ditions of stretch and fusimotor activation may be due to tor, together with about 1 cm of nerve, was mounted in a small chamber by differences in their respective mechanoelectric transducers tying each pole (near the capsule sleeve) to a small tungsten wire shaft or to mechanical properties of the intrafusal muscle support- connected to a servo-controlled Ling vibrator (model 108). The chamber was ing those endings. This study of isolated cat muscle spindles continuously perfused with oxygenated, modified Krebs’ solution (Poppele et al., 1979). The nerve was drawn onto a pair of electrodes in an adjacent examines the strain in individual intrafusal muscle fibers chamber containtng a high density fluorocarbon compound (FC-80, 3M Co.). resulting from stretch and fusimotor stimulation. The degree The entire assembly was mounted on a Zeiss photomicroscope equipped of local stretch occurring at the sensory endings under these with tine camera and Nomarski optics (see Poppele et al., 1979, and Poppele conditions was measured. -

Skeletal and Cardiac Muscle Pericytes: Functions and Therapeutic Potential
UCLA UCLA Previously Published Works Title Skeletal and cardiac muscle pericytes: Functions and therapeutic potential. Permalink https://escholarship.org/uc/item/99p1707r Authors Murray, Iain R Baily, James E Chen, William CW et al. Publication Date 2017-03-01 DOI 10.1016/j.pharmthera.2016.09.005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California JPT-06957; No of Pages 10 Pharmacology & Therapeutics xxx (2016) xxx–xxx Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Associate editor: P. Madeddu Skeletal and cardiac muscle pericytes: Functions and therapeutic potential Iain R. Murray a,b, James E. Baily a, William C.W. Chen c,AyeletDard, Zaniah N. Gonzalez a, Andrew R. Jensen d, Frank A. Petrigliano d, Arjun Deb e,⁎, Neil C. Henderson f,⁎⁎ a BHF Center for Vascular Regeneration and MRC Center for Regenerative Medicine, University of Edinburgh, Edinburgh, UK b Department of Trauma and Orthopaedic Surgery, The University of Edinburgh, Edinburgh, UK c Reseach Laboratory of Electronics and Department of Biological Engineering, Massachusetts Institute of Technology, Boston, MA, USA d Orthopedic Hospital Research Center, University of California, Los Angeles, CA, USA e Division of Cardiology, Department of Medicine & Molecular Cell and Developmental Biology, and Eli and Edythe Broad Institute of Regenerative Medicine and Stem Cell Research, David Geffen School of Medicine and College of Letters and Sciences, University of California, Los Angeles, CA, USA f MRC Centre for Inflammation Research, University of Edinburgh, Edinburgh, UK article info abstract Keywords: Pericytes are periendothelial mesenchymal cells residing within the microvasculature. Skeletal muscle and car- Perivascular stem cell diac pericytes are now recognized to fulfill an increasing number of functions in normal tissue homeostasis, in- Mesenchymal stem cell cluding contributing to microvascular function by maintaining vessel stability and regulating capillary flow. -

Cardiac Muscle: Structure and Properties
CARDIOVASCULAR SYSTEM: CARDIAC MUSCLE: STRUCTURE AND PROPERTIES For: Semester II CC2TH/ GEN 2TH Prepared and Compiled By: OLIVIA CHOWDHURY DEPARTMENT OF PHYSIOLOGY SURENDRANATH COLLEGE April 29, 2020 OLIVIA CHOWDHURY •Anatomy of The Heart April 29, 2020 OLIVIA CHOWDHURY •The Layers Of The Heart Three layers: • Epicardium . Pericardium – a double serous membrane . Visceral pericardium (Next to heart) . Parietal pericardium (Outside layer) . Serous fluid fills the space between the layers of pericardium . Connective tissue layer • Myocardium . Middle layer . Mostly cardiac muscle • Endocardium . Inner layer . Endothelium April 29, 2020 OLIVIA CHOWDHURY • The Heart Valves Allows blood to flow in only one direction Four valves: Atrioventricular valves– between atria and ventricles Bicuspid/ Mitral valve between LA and LV Tricuspid valve between RA and RV Semilunar valves between ventricles and arteries Pulmonary semilunar valve Aortic semilunar valve April 29, 2020 OLIVIA CHOWDHURY •Direction Of Blood Flow In The Heart April 29, 2020 OLIVIA CHOWDHURY Right side of the heart: • receives venous blood from systemic circulation via superior and inferior vena cava into right atrium • pumps blood to pulmonary circulation from right ventricle Left side of the Heart: • receives oxygenated blood from pulmonary veins • pumps blood into systemic circulation April 29, 2020 OLIVIA CHOWDHURY •The Cardiac Muscle Myocardium has three types of muscle fibers: Muscle fibers which form contractile unit of heart Muscle fibers which form the pacemaker Muscle fibers which form conductive system April 29, 2020 OLIVIA CHOWDHURY •The Cardiac Muscle Striated and resemble the skeletal muscle fibre Sarcomere is the functional unit Sarcomere of the cardiac muscle has all the contractile proteins, namely actin, myosin, troponin tropomyosin. -

MUSCLE TISSUE Larry Johnson Texas A&M University
MUSCLE TISSUE Larry Johnson Texas A&M University Objectives • Histologically identify and functionally characterize each of the 3 types of muscle tissues. • Describe the organization of the sarcomere as seen in light and electron microscopy. • Identify the endomysium, perimysium, and epimysium CT sleeves in muscle. • Relate the functional differences of the three muscle cell types. From: Douglas P. Dohrman and TAMHSC Faculty 2012 Structure and Function of Human Organ Systems, Histology Laboratory Manual MUSCLE FUNCTION: • GENERATION OF CONTRACTILE FORCE DISTINGUISHING FEATURES: • HIGH CONCENTRATION OF CONTRACTILE PROTEINS ACTIN AND MYOSIN ARRANGED EITHER DIFFUSELY IN THE CYTOPLASM (SMOOTH MUSCLE) OR IN REGULAR REPEATING UNITS CALLED SARCOMERES (STRIATED MUSCLES, e.g., CARDIAC AND SKELETAL MUSCLES) MUSCLE • DISTRIBUTION: SKELETAL – STRIATED MUSCLES MOSTLY ASSOCIATED WITH THE SKELETON MUSCLE • DISTRIBUTION: SKELETAL – STRIATED MUSCLES MOSTLY ASSOCIATED WITH THE SKELETON CARDIAC – STRIATED MUSCLES ASSOCIATEWD WITH THE HEART MUSCLE • DISTRIBUTION: SKELETAL – STRIATED MUSCLES MOSTLY ASSOCIATED WITH THE SKELETON CARDIAC – STRIATED MUSCLES ASSOCIATEWD WITH THE HEART SMOOTH – FUSIFORM CELLS ASSOCIATED WITH THE VISCERA, RESPIRATORY TRACT, BLOOD VESSELS, UTERUS, ETC. MUSCLE • HISTOLOGICAL INDENTIFICATION: SKELETAL MUSCLE – VERY LONG CYLINDRICAL STRIATED MUSCLE CELLS WITH MULTIPLE PERIPHERAL NUCLEI MUSCLE • HISTOLOGICAL INDENTIFICATION: SKELETAL MUSCLE – VERY LONG CYLINDRICAL STRIATED MUSCLE CELLS WITH MULTIPLE PERIPHERAL NUCLEI CARDIAC MUSCLE – -

Spinal Reflexes
Spinal Reflexes Lu Chen, Ph.D. MCB, UC Berkeley 1 Simple reflexes such as stretch reflex require coordinated contraction and relaxation of different muscle groups Categories of Muscle Based on Direction of Motion Flexors Æ reduce the angle of joints Extensors Æ increase the angle of joints Categories of Muscle Based on Movement Agonist Æmuscle that serves to move the joint in the same direction as the studied muscle Antagonist Æ muscle that moves the joint in the opposite direction 2 1 Muscle Spindles •Small encapsulated sensory receptors that have a Intrafusal muscle spindle-like shape and are located within the fibers fleshy part of the muscle •In parallel with the muscle fibers capsule •Does not contribute to the overall contractile Sensory force endings •Mechanoreceptors are activated by stretch of the central region Afferent axons •Due to stretch of the whole muscle Efferent axons (including intrafusal f.) •Due to contraction of the polar regions of Gamma motor the intrafusal fibers endings 3 Muscle Spindles Organization 2 kinds of intrafusal muscle fibers •Nuclear bag fibers (2-3) •Dynamic •Static •Nuclear chain fibers (~5) •Static 2 types of sensory fibers •Ia (primary) - central region of all intrafusal fibers •II (secondary) - adjacent to the central region of static nuclear bag fibers and nuclear chain fibers Intrafusal fibers stretched Sensory ending stretched, (loading the spindle) increase firing Muscle fibers lengthens Sensory ending stretched, (stretched) increase firing Spindle unloaded, Muscle fiber shortens decrease firing 4 2 Muscle Spindles Organization Gamma motor neurons innervate the intrafusal muscle fibers. Activation of Shortening of the polar regions gamma neurons of the intrafusal fibers Stretches the noncontractile Increase firing of the center regions sensory endings Therefore, the gamma motor neurons provide a mechanism for adjusting the sensitivity of the muscle spindles.